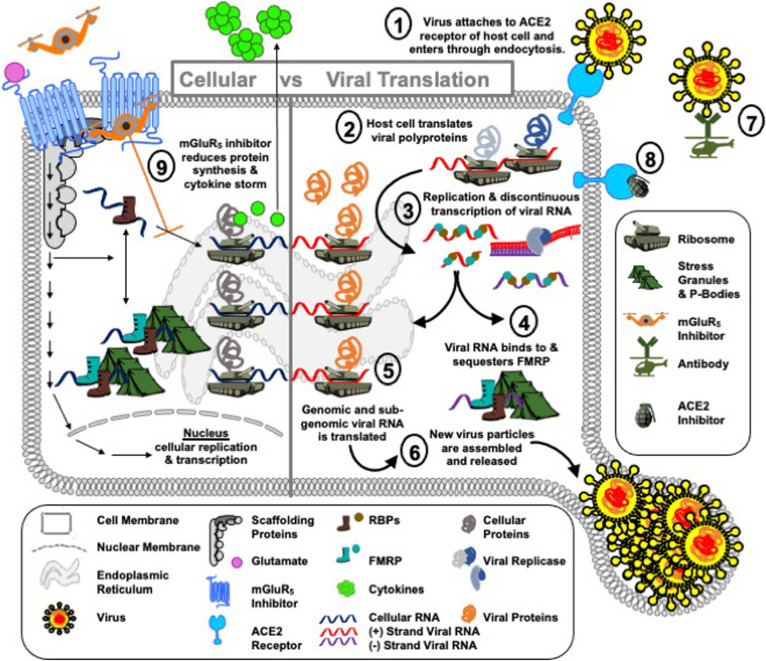
FIGURE 4.
Operation mGluR5. COVID-19 is a global pandemic, i.e., a Blitzkrieg attack by SARS-CoV-2 on the human population. The port of attack for SARS-CoV-2 is the cell surface receptor angiotensin-converting enzyme 2 (ACE2) in the cells of the lungs ➀. Once SARS-CoV-2 lands and breaches the cell border, the virus injects positive-sense, single-stranded RNA [ssRNA(+)] into the cell cytoplasm and immediately takes hostage of the host cell protein synthesis machinery ➁ to replicate and transcribe new viral RNA ➂. This is accomplished by a swift and effective disarmament of the cell’s shock troops, RNA binding proteins (RBPs). Shock troops is a military term for infantry formations created to lead an attack. In the RNA world, RBPs bind to RNA to either degrade, localize, store or translate messages. RBPs can bind to viral as well as cellular mRNA. In the case of viral infection, viral RNA recruits cellular RBPs to translate viral proteins at ribosomes, or sequesters cellular RBPs at other cell encampments, such as stress granules and P-bodies, to block their normal cellular function. We hypothesize that FMRP is a shock troop that is sequestered by SARS-CoV-2 RNA to prevent its normal function of acting as a brake on protein synthesis ➃. In the absence of FMRP, it is predicted that the rate of viral protein synthesis ➄ and hence further infection ➅ are increased. Public surveillance policies and on-site diagnostics (i.e., equivalent to wartime communications and intelligence reports) to inform the public and health care professionals on viral spread are in place. Vaccines, which can mediate a rapid immune response to fight infection are in progress (i.e., cell airborne attack) ➆. Drugs to target ACE2, the port of infection, are identified and under study (i.e., cell naval response) ➇. What we lack in the fight against SARS-CoV-2 are drugs that support the boots on the ground, i.e., RBPs, and protect their encampments, i.e., ribosomes, stress granules and P-bodies. We propose that mGluR5 inhibitors ➈ are a potential drug therapy to combat viral hijack of the host translational infrastructure (i.e., the cell army) by slowing down protein synthesis to afford the innate immune system time to identify a viral infection and mediate an adaptive response as well as to afford the cell degradation machinery (i.e., cell marines) time to recruit and degrade viral proteins. It is anticipated that reduced protein synthesis could have negative consequences for the host cell as well as the virus; however, similar to chemotherapy that kills both healthy and cancer cells, this defensive strategy to delay advance of the stealth virus invader could buy time until the enemy can be eradicated by flanking troops. An additional potential benefit of mGluR5 inhibition is reduced cytokine production, which could attenuate the COVID-19 cytokine storm.