Abstract
Introduction
Induced pluripotent stem cell (iPSC)-derived endothelial cells (ECs) have the potential for therapeutic application in several cardiovascular diseases. Mechanical strain is known to regulate EC behavior and stem cell differentiation and may play a role in directing EC differentiation of iPSCs. H19, a long non-coding RNA (lncRNA), is known to affect ECs in several mechanically relevant pathologies and may play a role in this process as well. Therefore, we investigated expression changes of H19 resulting from mechanical stimulation during EC differentiation, as well as functional effects on EC tube formation.
Methods
iPSCs were subjected to 5% cyclic mechanical strain during EC differentiation. RT-PCR and flow cytometry were used to assess changes in mesoderm differentiation and gene expression in the final ECs as a result of strain. Functional outcomes of mechanically differentiated ECs were assessed with a tube formation assay and changes in H19. H19 was also overexpressed in human umbilical vein endothelial cells (HUVECs) to assess its role in non-H19-expressing ECs.
Results
Mechanical strain promoted mesoderm differentiation, marked by increased expression of brachyury 24 h after initiation of differentiation. Strain also increased expression of H19, CD31, VE-cadherin, and VEGFR2 in differentiated ECs. Strain-differentiated ECs formed tube networks with higher junction and endpoint density than statically-differentiated ECs. Overexpression of H19 in HUVECs resulted in similar patterns of tube formation.
Conclusions
H19 expression is increased by mechanical strain and promotes tube branching in iPSC-derived ECs.
Keywords: lncRNA, Angiogenesis, Mechanobiology, Stem cells
Introduction
Endothelial cells (ECs) line the interior of the circulatory system and are critically involved in a variety of cardiovascular functions and diseases. During healthy function, ECs regulate nutrient and oxygen exchange, mediate immune cell infiltration from the circulatory system to target tissues, maintain vascular tone, and are crucial for the formation of new blood vessels.24 The endothelium is also directly involved in numerous cardiovascular diseases, such as atherosclerosis, coronary artery disease, or valvular heart diseases, and induced pluripotent stem cell (iPSC)-derived ECs are increasingly being considered for cell therapy approaches to replace the dysfunctional endothelium. Therefore, therapeutic success with iPSC-derived ECs will require a detailed understanding of the factors that influence EC differentiation and the functional impacts on the final cells.
Several methods to generate iPSC-derived ECs have been reported.11,15,18,23 Protocols vary, but the progression from pluripotency through a mesodermal lineage with subsequent EC specification and purification is quite consistent. Typically, this is done with though activation of the Wnt pathway using a GSK3 inhibitor, followed by endothelial specification using a high levels of VEGF. Several studies demonstrate methods to generate subtype-specific ECs including arterial and venous ECs, blood brain barrier ECs, and endocardial cells. These approaches have largely relied on alterations in the makeup, concentration, and timing of the delivery of soluble factors which drive the differentiation process. While soluble signaling will likely remain a key component of iPSC differentiation, additional factors such as the mechanical environment have also been shown to affect stem cell differentiation and should be better understood for a complete picture of EC differentiation.
Passive mechanical cues such as cell substrate stiffness, stiffness gradient, and cellular shape confinement have been shown to direct stem cell fate towards different lineages.5,13 Active mechanical stimulation, including cyclic strain and fluid shear have also been investigated as regulators of stem cell differentiation, with mixed results.1,28,31 Strain has previously been shown to promote stem cell pluripotency, though other studies have found strain to promote smooth muscle cell differentiation.26,28 In the context of endothelial development, fluid shear stress has been the major focus of in vivo developmental studies, as well as most in vitro work, and it has often been shown that low levels of fluid shear promote an endothelial phenotype.20,25 In this study, we investigate the role of cyclic mechanical strain, such as would be imparted by cardiac contraction on the differentiation of iPSC-derived ECs.
Several genes known to regulate EC development such as NOTCH1 and members of the Wnt family are reexpressed in states of cardiovascular disease, particularly pathologies characterized by extensive remodeling of the extracellular matrix and alterations to the mechanical environment such as atherosclerosis and calcific aortic valve disease.2–4,7,21,22 Recently, long non-coding RNA (lncRNA) H19 has been implicated as a potential driver of such diseases.8–10H19 is a developmentally expressed lncRNA that is imprinted after birth, with very low expression in most mature tissues.6,29H19’s reemergence in disease states suggests a similarity to other developmentally active genes, and crosstalk between H19 and many developmental players has been demonstrated in disease states. For example, H19 represses NOTCH1 transcription and promotes BMP2 activity in calcific aortic valve disease, and increased H19 activates Wnt/β-Catenin signaling and mediating BMP9 activity in osteoblast differentiation and glioma.8,12,16,17 These interactions make H19 a promising candidate for cardiovascular research and prompted our interest in it for this study. Furthermore, it’s involvement in mechanically altered disease states raises questions about its mechanosensitivity, which to date has not been clearly demonstrated.
The aim of this study was to investigate the role of mechanical forces on EC differentiation of iPSCs, specifically focusing on H19 expression and downstream effects. The effects of mechanical strain were examined in the context of mesoderm differentiation of iPSCs as well as the expression profile and functional changes to the resultant ECs. Functional effects of alterations in H19 expression were assessed using an H19 overexpression model in human umbilical vein endothelial cells (HUVECs), in which H19 was normally minimally expressed.
Methods
Cell Culture
Human iPSCs (line DF19-9-11T, WiCell, male donor) were maintained on six-well plates coated with growth factor reduced Matrigel (Corning) in mTeSR media (StemCell Technologies) changed daily and passaged with ReLeSR passaging reagent (StemCell Technologies) according to manufacturer’s instructions. All experiments were performed with cells between passage 30 and 50. HUVECs (ATCC, pooled donors) were maintained on gelatin coated dishes in EGM-2 (Lonza) and passaged with Trypsin at confluence. All HUVEC experiments were performed with cells between passage six and ten.
Endothelial Cell Differentiation
EC differentiations were carried out as described by Patsch et al., with minor modifications to allow for cell adhesion and differentiation under strain conditions.23 Prior to differentiation, untreated BioFlex plates (Flexcell International Corp.) were coated with 10 μg/cm2 growth factor reduced Matrigel diluted in Dulbecco’s Modified Eagle’s Medium/Ham’s F-12 (DMEM/F12) (Gibco) and incubated at 37 °C for a minimum of 4 h. iPSCs were allowed to reach 90% confluency and passaged with ReLeSR onto the coated BioFlex plates at a 1:10 split ratio in mTeSR with 10 μM ROCK inhibitor Y-27632 (Tocris Biosciences). Cell clump size during passaging was kept at 10–30 cells per clump to ensure cell adhesion and survival during differentiation. After 24 h, media was changed to N2B27 mesoderm induction media, consisting of a 50:50 mixture of DMEM/F12 and neurobasal media (Gibco) supplemented with N2 (Gibco), B27 (Gibco), 7 μM CHIR99021 (Tocris) and 25 ng/mL BMP4 (Peprotech). At this point, cells undergoing strain were subjected to 5% equibiaxial strain on the FT-4000 tension system (Flexcell International Corp.) at a duty cycle of 1 Hz for the duration of the differentiation while statically-differentiated cells were differentiated on unstrained BioFlex plates. This strain rate falls within biologically relevant ranges, and was experimentally found to be the highest magnitude strain that could be applied without significant cellular detachment. After 72 h without media change, media was replaced with endothelial induction media, consisting of StemPro-34 SFM medium (Gibco) with 200 ng/mL VEGF (Genscript) and 2 μM Forskolin (Tocris), changed daily. After 48 h of endothelial differentiation, cells were detached with Accutase (Innovative Cell Technologies) and enriched for endothelial cells using magnetic cell separation for VE-cadherin-positive cells, following manufacturer’s instructions (Miltenyi Biotec). VE-cadherin-positive cells were either used immediately for RT-PCR or plated in StemPro-34 supplemented with 50 ng/mL VEGF onto dishes coated with human fibronectin (Corning) for later use in Matrigel tube assays.
RT-PCR
Total RNA was purified from pelleted cells using Trizol Reagent (Invitrogen) according to the manufacturer’s instructions. For HUVEC transfection experiments, RNA was purified from the Trizol using a Direct-zol RNA MiniPrep kit (Zymo Research) to allow for DNAse treatment to ensure removal of remaining plasmid DNA. Equivalent amounts of RNA were reverse transcribed using the Superscript IV First-Strand Synthesis System (Life Technologies) and the supplied oligo(dT) primers. RT-PCR was performed with equal amounts of cDNA using iQ SYBR Green Supermix (Bio-Rad) and gene specific primers listed in Table 1. Gene expression was normalized to GAPDH.
Table 1.
RT-PCR primer sequences.
| Gene name | Forward primera | Reverse primera |
|---|---|---|
| Gapdh | CAGCCTCAAGATCATCAGCA | ATCCACAGTCTTCTGGGTGG |
| H19 | ACACAAAACCCTCTAGCTTGG | GTCTTTGATGTTGGGCTGATG |
| CD31 | TCTGATTGGCTAACTGAACCC | CAGACACCATTCCAAAACCAG |
| VE-cadherin | CAGATCTCCGCAATAGACAAGG | TATGCTCCCGGTCAAACTG |
| VEGFR2 | TCTTTTGGTGTTTTGCTGTGG | TGGTCTGGTACATTTCTGGTG |
aPrimer sequences are from 5′ to 3′
Flow Cytometry
Cells were dissociated with Accutase and collected in FACS buffer consisting of 3% fetal bovine serum in Dulbecco’s phosphate buffered saline (DPBS). Cells were fixed in 2% formaldehyde in PBS for 30 min at room temperature, then pelleted and permeabilized in 0.1% Triton-X for 15 min at room temperature. Primary antibody against brachyury (Cell Signaling Technology, #81694, clone D2Z3J) and fluorescently labeled secondary antibody (ThermoFisher, A21245, Lot 1837984) were then used to label the cells, which were then analyzed on a 3-laser LSR-II analyzer.
Tube Formation Assay
Growth factor reduced Matrigel was thawed on ice, and 125 μL per well was added to a 48-well plate and allowed to gel for 30 min at 37 °C. ECs (either transfected HUVECs or iPSC-derived ECs) were dissociated with Accutase and seeded on top of the gels at 40,000 cells per well in 200 μL of the respective growth media. After 4 or 8 h (for HUVECs and iPS-ECs respectively), media was replaced with 200 μL of media containing 2 μM Calcein-AM (ThermoFisher Scientific) for 20 min. Gels were then fluorescently imaged using a 488 nm excitation wavelength. Full frame images were cropped for regions in focus and analyzed using AngioTool.33 All metrics were normalized to image area. Data came from 3 to 4 images per gel of two gels seeded from four independent differentiations or transfections.
Statistics
All data are presented as mean ± standard error. Statistical significance of RT-PCR fold change was assessed with a one-sided t-test on normalized Ct values, and statistical significance of tube assay formation metrics was assessed with one-way ANOVA with Tukey’s post-hoc test. In the event that data were not normally distributed, significance was assessed using ANOVA on ranks. p < 0.05 was considered to be significant.
Results
EC Differentiation of iPSCs is Possible on FlexCell Plates with Cyclic Strain
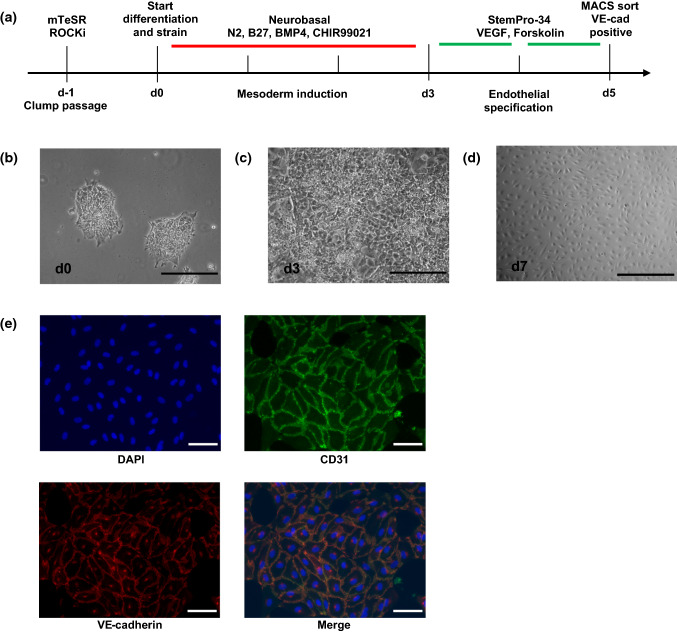
Using minor modifications to a previously published endothelial differentiation protocol, we were able to differentiate iPSCs to a vascular endothelial lineage (Fig. 1a). To allow for more robust adhesion to the FlexCell plates and continued attachment during application of cyclic strain, plates were incubated in Matrigel coating media overnight and iPSCs were seeded as clumps of 10–30 cells rather than single cells (Fig 1b). At the start of mesoderm induction, cells were also subjected to 5% cyclic, equibiaxial strain at a 1 Hz duty cycle. After 72 h, a dense monolayer similar to previously reported protocols was obtained, and media was switched to VEGF-containing endothelial induction media, changed daily (Fig 1c). ECs were enriched by MACS sorting for vascular endothelial cadherin (VE-cadherin), and the resulting cell populations formed typical endothelial monolayers (Fig 1d) and stained positively for CD31 and VE-cadherin (Fig 1e).
Figure 1.
Endothelial differentiation of iPSCs on FlexCell plates. iPSCs were differentiated to ECs according to the timeline in (a). Cells were passaged as clumps to promote adhesion to the flexible substrate (b). After 3 days of mesoderm induction, cells formed a confluent monolayer. Following 2 days of endothelial specification and magnetic sorting for VE-cadherin positive cells, purified ECs formed traditional endothelial monolayers and stained positively for CD31 and VE-cadherin (d, e). Scale bar = 500 μm in B,C,D; scale bar = 100 μm in (e).
Strain Accelerates Mesoderm Induction and H19 Expression
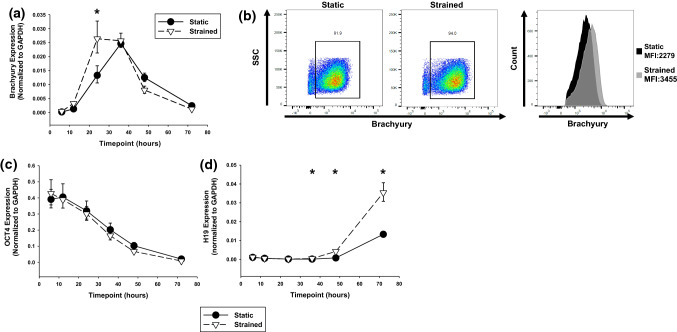
Knowing that mechanical forces are capable of affecting stem cell differentiation, we also probed the effects on mesoderm differentiation and found an accelerated shift from pluripotency to a mesoderm lineage, marked by elevated transcription of brachyury at 24 h with strain compared to statically-differentiated cells (Fig. 2a, 24 h p = 0.047). This finding was investigated at the translational level with flow cytometry, which showed minimal change in percentage of brachyury-positive cells at 24 h, though a robust increase in median fluorescence intensity in the strain-differentiated cells compared with the statically-differentiated, suggesting higher levels of brachyury translation per cell (Fig. 2b). In both conditions, brachyury transcription peaked between 24 and 36 h before a strong decrease prior to endothelial induction. Mesoderm differentiation and loss of pluripotency was further confirmed by a loss of OCT4 expression over the duration of mesoderm specification (Fig. 2c). Interestingly, H19 expression was also found to be quite low during initial stages of mesoderm differentiation, but increased dramatically by 48 and 72 h (Fig. 2d).
Figure 2.
Mesoderm differentiation of iPSCs with strain. Strain accelerated mesoderm differentiation, marked by increased expression of brachyury at 24 h after initiation of differentiation (a, p = 0.047). Flow cytometry showed a minimal increase in brachyury positive cells and modest increase in fluorescence intensity in strained cells (b). Mesoderm differentiation was also marked by loss of pluripotency marker OCT4 (c). Strain increased expression of H19 by 36 h from the start of differentiation (p = 0.014) and continuing to increase at 48 h (p = 0.008 and 72 h (p = 0.002) (d). SSC side scatter; *p < 0.05 by ANOVA.
Strain Induces Transcriptional Increase in H19 in iPS-Derived ECs
After VE-cadherin enrichment, the resultant ECs were assessed for transcriptional changes. As with mesoderm differentiation, cyclic strain induced 2.2-fold higher expression of H19 in fully differentiated iPSC-ECs (Fig. 3a, p = 0.012). This was also accompanied by higher transcription of endothelial markers CD31, VE-cadherin, and VEGF receptor 2 (VEGFR2) (Fig. 3a, p = 0.0006, p = 0.0009, p = 0.036 respectively).
Figure 3.
Strain increased expression of H19, CD31, VE-cadherin, and VEGFR2 in differentiated endothelial cells (a, p = 0.0123, p = 0.0006, p = 0.0009, p = 0.0363 respectively). Strain-differentiated and statically-differentiated ECs were used in a Matrigel tube assay and analyzed with AngioTool (b). Top panels show Calcein-AM stained tube networks (green) and bottom panels show AngioTool analysis overlay (red = tube, blue = junction). Strain-differentiated iPSC-derived ECs formed tube networks with higher junction density, lower average vessel length, and higher endpoint density (c–e, p = 0.002, p = 0.020, p = 0.035 respectively). Data points from 3 to 4 images per gel of two gels seeded from each of four independent differentiations. Scale bar = 500 μm; *p < 0.05 by ANOVA; #p < 0.05 by ANOVA on ranks.
Strain-Differentiated ECs Exhibit Higher Endothelial Tube Junction Formation
Functional outcomes of the increased levels of H19 and other endothelial markers were tested using a Matrigel tube assay. Strain-differentiated ECs were found to compact into tube-like structures more quickly, such that at 4 h post-seeding, the majority of the cells had formed tubes. Conversely, statically-differentiated ECs were slower to form tubes and at 4 h were seen to remain in monolayer on the surface of the Matrigel in many areas of the well. By 8 h, both conditions had compacted into more mature tube-like structures (Fig. 3b). Analysis of tube networks showed that strain-differentiated ECs had higher junction density, resulting in shorter average tube length and higher endpoint density (Figs. 3b–3e).
H19 is a Driver of Increased Endothelial Tube Junction Formation in HUVECs
To assess the role of H19 in the observed increase in tube formation in strain-differentiated ECs, HUVECs were transfected with an H19 overexpression construct as a proxy for strain-induced H19 upregulation. Overexpression was confirmed with RT-PCR and at levels comparable to iPSC-derived ECs; H19 was unexpressed in HUVECs transfected with empty vector control plasmid (Fig. 4a). Expression of other EC markers was not altered by overexpression of H19 I Fig. 4a). In a tube formation assay with HUVECs, H19 overexpression was found to mirror the effects seen in strain-differentiated iPSC-derived ECs, namely, increased junction density and endpoint density with shorter average tube length (Figs. 4b–4e).
Figure 4.
H19 expression in HUVECs replicates strain-differentiation in a tube assay. HUVECs were transfected with either a pcDNA3.1 H19 overexpression construct or empty pcDNA3.1 as a control. H19 was found to be highly expressed in the overexpression condition and was not detected in control transfections, while CD31 and VE-cadherin expression were unchanged (a, p < 0.001). As with iPSC-derived ECs, H19 expression led to more compact tube formation (b) with higher junction density, lower average tube length, and higher endpoint density (c–e), p = 0.003, p = 0.005, p < 0.001 respectively). Data points from 3 to 4 images per gel of two gels seeded from each of four independent transfections. Scale bar = 500 μm; green = calcein-AM; red = identified tube; blue = identified junction; *p < 0.05 by ANOVA; #p < 0.05 by ANOVA on ranks.
Discussion
This study demonstrates a role for active mechanical force in iPSC-derived EC differentiation and H19 expression as well as a functional outcome of increased H19 in ECs. While other studies have shown crosstalk between mechanical forces and H19 expression in various in vivo disease contexts, this is the first study to investigate this relationship in EC differentiation of iPSCs. By applying cyclic, equiaxial strain to differentiating iPSCs, mesoderm differentiation was accelerated, as shown by increased expression of mesoderm marker brachyury. Over the same timeframe, strain led to increased expression of H19, which persisted through EC differentiation, leading to increased tube formation in a Matrigel tube assay.
We found that mechanical strain induces mesoderm differentiation of iPSCs, shown by accelerated expression of mesoderm marker brachyury. Interestingly, other studies have shown that strain over 10% acts to maintain pluripotency and prevent spontaneous differentiation of human embryonic stem cells in conditioned (pluripotency maintenance) media.26 However, lower strain rates and application of unconditioned, differentiation-permitting media did not act to prevent differentiation, suggesting a synergistic effect of both chemical stimuli and mechanics in the induction of differentiation. The present work reinforces this concept and shows that in the context of chemically driven EC differentiation, strain actually serves to promote earlier mesoderm differentiation, eventually leading to increased expression of traditional endothelial markers.
One of the major findings of this work was the impact of strain during differentiation on the ability of iPSC-derived ECs to form tube networks. Mechanical forces have long been known to affect vascular network formation and have been studied developmentally and with a variety of in vitro methods. Generally, physiologically relevant mechanical stimuli are thought to promote vasculogenesis, though most studies show this relationship using fully differentiated ECs or in vivo developmental models, as opposed to our investigation of strain applied prior to endothelial tube formation.20,25 One investigation of strain applied to differentiating embryoid bodies found that strain promoted vasculogenesis, suggesting a role for mechanical stimuli earlier in the developmental process.27 In particular, strain was shown to promote branching points. Our study corroborates these findings, showing that strain applied during endothelial differentiation primes cells to form tube networks with increased branching, with evidence that H19 may mediate this effect.
Intersections between various mechanical stimuli and H19 expression have been previously shown, including in a pressure overload model of cardiac hypertrophy, a hindlimb unloading model of osteoporosis, and tension induced osteogenesis.14,19,30 In these studies, strain correlated with expression of H19. Similarly, H19 is known to be upregulated in calcific aortic valve disease, a pathology characterized by increased valvular strain which augments valve calcification.8 Our work shows a similar upregulation of H19 expression with increased mechanical strain. While some studies suggest that loss of imprint and upregulation of H19 expression may be driving certain cardiovascular diseases, our work indicates that alterations in the mechanical environment may precede H19 dysregulation. Such a mechanism would explain the occurrence of H19 dysregulation in many mechanically altered pathologies, and could be probed in otherwise healthy instances of disturbed mechanics. In any case, H19 has been shown to have downstream functional impact in both in vivo and in vitro models, and a thorough understanding of how the mechanical environment impacts its expression will be necessary for development cell-based or other therapies.
Our results show that along with increasing H19 expression, mechanical strain during endothelial differentiation also impacts endothelial tube formation, though further work is needed to demonstrate whether this pattern is causal or coincidental. Anecdotally, H19 expression was found to decrease in iPSC-derived ECs after 2–3 passages, suggesting the development of a genomic imprint that is common in most mature tissues. However, given our interest in H19 specific effects, as well as the high potential for morphological activity during early developmental stages, we used iPSC-ECs one passage after sorting to allow for the removal of non-adherent cells while maintaining high H19 expression. Direct modulation of H19 expression in iPSCs and iPSC-ECs was attempted, but was not consistently feasible, leading us to utilize HUVECs as a proxy cell model to investigate functional impacts of H19 expression in ECs. Because HUVECs do not normally express H19, they presented a cellular “blank slate” with which to study the isolated effects of H19 expression. Using this system, it was clear that increased H19 expression lead to similar tube formation trends in HUVECs, suggesting that H19 is a potential mediator of these changes in iPSC-derived ECs.
Previous studies have identified H19 as a regulator of angiogenesis and tube formation. One study of glioma-associated endothelial cells showed that H19 promotes angiogenesis, potentially through suppression of microRNA-29a, though work points to microRNA-181a as a more likely target in microvascular ECs.12,32 While these studies have shown potential downstream mechanisms by which H19 expression may enhance tube formation, our work extends potential explanations on the upstream side of this process. Strain-induced H19 expression may be a means by which vessel branching is initiated when hemodynamic demands exceed vessel capacity, though further experimentation in vivo is needed to demonstrate this.
This study shows that cyclic strain promotes earlier mesoderm differentiation of iPSCs and that ECs differentiated under strain conditions have increased branching in a tube formation assay. H19 was investigated as a mediator of this effect and was found to have similar functional outcomes in an overexpression model in HUVECs. While further work is needed to clearly identify the downstream effects of H19 expression in iPSC-derived ECs, it is clear that the mechanical environment impacts EC function and expression of H19.
Acknowledgments
Funding
This work was supported by the National Heart, Lung, and Blood Institute (HL135790 and HL007411) and the Fondation Leducq.
Conflict of interest
No conflict of interest, financial or otherwise, are declared by Dr. Vander Roest or Dr. Merryman.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahsan T, Nerem RM. Fluid shear stress promotes an endothelial-like phenotype during the early differentiation of embryonic stem cells. Tissue Eng. Part A. 2010;16:3547–3553. doi: 10.1089/ten.tea.2010.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai J, Pardali E, Sánchez-Duffhues G, ten Dijke P. BMP signaling in vascular diseases. FEBS Lett. 2012;586:1993–2002. doi: 10.1016/j.febslet.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 3.Dejana E. The role of wnt signaling in physiological and pathological angiogenesis. Circ. Res. 2010;107:943–952. doi: 10.1161/CIRCRESAHA.110.223750. [DOI] [PubMed] [Google Scholar]
- 4.Dyer LA, Pi X, Patterson C. The role of BMPs in endothelial cell function and dysfunction. Trends Endocrinol. Metab. 2014;25:472–480. doi: 10.1016/j.tem.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 6.Gabory A, Jammes H, Dandolo L. The H19 locus: role of an imprinted non-coding RNA in growth and development. BioEssays. 2010;32:473–480. doi: 10.1002/bies.200900170. [DOI] [PubMed] [Google Scholar]
- 7.Gridley T. Notch signaling in vascular development and physiology. Development. 2007;134:2709–2718. doi: 10.1242/dev.004184. [DOI] [PubMed] [Google Scholar]
- 8.Hadji F, et al. Altered DNA methylation of long noncoding RNA H19 in calcific aortic valve disease promotes mineralization by silencing NOTCH1. Circulation. 2016;134:1848–1862. doi: 10.1161/CIRCULATIONAHA.116.023116. [DOI] [PubMed] [Google Scholar]
- 9.Han DK, Khaing ZZ, Pollock RA, Haudenschild CC, Liau G. H19, a marker of developmental transition, is reexpressed in human atherosclerotic plaques and is regulated by the insulin family of growth factors in cultured rabbit smooth muscle cells. J. Clin. Invest. 1996;97:1276–1285. doi: 10.1172/JCI118543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofmann P, et al. Long non-coding RNA H19 regulates endothelial cell aging via inhibition of STAT3 signalling. Cardiovasc. Res. 2019;115:230–242. doi: 10.1093/cvr/cvy206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James D, et al. Expansion and maintenance of human embryonic stem cell – derived endothelial cells by TGFb inhibition is Id1 dependent. Nat. Biotechnol. 2010;28:1–7. doi: 10.1038/nbt.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia P, et al. Long non-coding RNA H19 regulates glioma angiogenesis and the biological behavior of glioma-associated endothelial cells by inhibiting microRNA-29a. Cancer Lett. 2016;381:359–369. doi: 10.1016/j.canlet.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Placone JK, Engler AJ. Understanding the extracellular forces that determine cell fate and maintenance. Development. 2017;144:4261–4270. doi: 10.1242/dev.158469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li B, et al. LncRNA-H19 modulates Wnt/β-catenin signaling by targeting Dkk4 in hindlimb unloaded rat. Orthop. Surg. 2017 doi: 10.1111/os.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lian X, et al. Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling. Stem Cell Rep. 2014;3:804–816. doi: 10.1016/j.stemcr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang W-C, et al. H19 activates Wnt signaling and promotes osteoblast differentiation by functioning as a competing endogenous RNA. Sci. Rep. 2016;6:20121. doi: 10.1038/srep20121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao J, et al. lncRNA H19 mediates BMP9-induced osteogenic differentiation of mesenchymal stem cells (MSCs) through Notch signaling. Oncotarget. 2017;8:53581–53601. doi: 10.18632/oncotarget.18655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Y, Gil C-H, Yoder MC. Differentiation, evaluation, and application of human induced pluripotent stem cell-derived endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2017;37:2014–2025. doi: 10.1161/ATVBAHA.117.309962. [DOI] [PubMed] [Google Scholar]
- 19.Liu L, et al. The H19 long noncoding RNA is a novel negative regulator of cardiomyocyte hypertrophy. Cardiovasc. Res. 2016;111:56–65. doi: 10.1093/cvr/cvw078. [DOI] [PubMed] [Google Scholar]
- 20.Lu D, Kassab GS. Role of shear stress and stretch in vascular mechanobiology. J. R. Soc. Interface. 2011;8:1379–1385. doi: 10.1098/rsif.2011.0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacGrogan D, Münch J, de la Pompa JL. Notch and interacting signalling pathways in cardiac development, disease, and regeneration. Nat. Rev. Cardiol. 2018;15:685–704. doi: 10.1038/s41569-018-0100-2. [DOI] [PubMed] [Google Scholar]
- 22.Pahnke A, et al. The role of Wnt regulation in heart development, cardiac repair and disease: a tissue engineering perspective. Biochem. Biophys. Res. Commun. 2016;473:698–703. doi: 10.1016/j.bbrc.2015.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patsch C, et al. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat. Cell Biol. 2015;17:994–1003. doi: 10.1038/ncb3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajendran P, et al. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013;9:1057–1069. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Resnick N, et al. Fluid shear stress and the vascular endothelium: for better and for worse. Prog. Biophys. Mol. Biol. 2003;81:177–199. doi: 10.1016/s0079-6107(02)00052-4. [DOI] [PubMed] [Google Scholar]
- 26.Saha S, Ji L, de Pablo JJ, Palecek SP. Inhibition of human embryonic stem cell differentiation by mechanical strain. J. Cell. Physiol. 2006;206:126–137. doi: 10.1002/jcp.20441. [DOI] [PubMed] [Google Scholar]
- 27.Sharifpanah F, Behr S, Wartenberg M, Sauer H. Mechanical strain stimulates vasculogenesis and expression of angiogenesis guidance molecules of embryonic stem cells through elevation of intracellular calcium, reactive oxygen species and nitric oxide generation. Biochim. Biophys. Acta. 2016;1863:3096–3105. doi: 10.1016/j.bbamcr.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu N, et al. Cyclic strain induces mouse embryonic stem cell differentiation into vascular smooth muscle cells by activating PDGF receptor β. J. Appl. Physiol. 2008;104:766–772. doi: 10.1152/japplphysiol.00870.2007. [DOI] [PubMed] [Google Scholar]
- 29.Thorvaldsen JL, Fedoriw AM, Nguyen S, Bartolomei MS. Developmental profile of H19 differentially methylated domain (DMD) deletion alleles reveals multiple roles of the DMD in regulating allelic expression and DNA methylation at the imprinted H19/Igf2 locus. Mol. Cell. Biol. 2006;26:1245–1258. doi: 10.1128/MCB.26.4.1245-1258.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu J, et al. Long non-coding RNA H19 mediates mechanical tension-induced osteogenesis of bone marrow mesenchymal stem cells via FAK by sponging miR-138. Bone. 2018;108:62–70. doi: 10.1016/j.bone.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto K, et al. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am. J. Physiol. Circ. Physiol. 2005;288:H1915–H1924. doi: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- 32.Zhu A, Chu L, Ma Q, Li Y. Long non-coding RNA H19 down-regulates miR-181a to facilitate endothelial angiogenic function. Artif. Cells Nanomed. Biotechnol. 2019;47:2698–2705. doi: 10.1080/21691401.2019.1634577. [DOI] [PubMed] [Google Scholar]
- 33.Zudaire E, Gambardella L, Kurcz C, Vermeren S. A computational tool for quantitative analysis of vascular networks. PLoS ONE. 2011;6:e27385. doi: 10.1371/journal.pone.0027385. [DOI] [PMC free article] [PubMed] [Google Scholar]