Table 1.
Bile acid agonists and its derivatives.
| Name | Chemical structures | Preclinical studies | Current clinical status | References |
|---|---|---|---|---|
| Obeticholic acid (OCA, INT-747) |
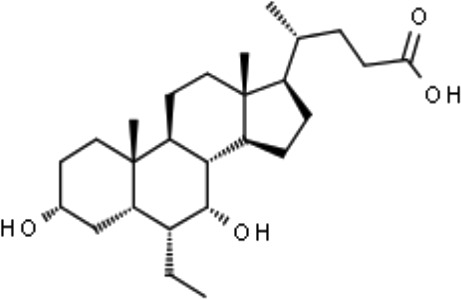
|
NASH mice; estrogen-induced cholestasis; rodent models of cholestasis. |
Phase II for Lipodystrophy (NCT02430077); Phase IV for Primary Biliary Cholangitis (NCT02308111) | (Fiorucci et al., 2005; Carino et al., 2020; Li et al., 2020) |
| INT-767 |
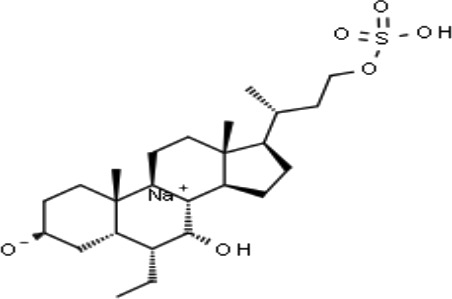
|
Chronic cholangiopathy (Mdr2-/- Abcb4-/- mice); Lepob/ob mice with NASH; HFD induced metabolic disorders. | NA | (Baghdasaryan et al., 2011; Jadhav et al., 2018; Roth et al., 2018) |
| TC-100 |
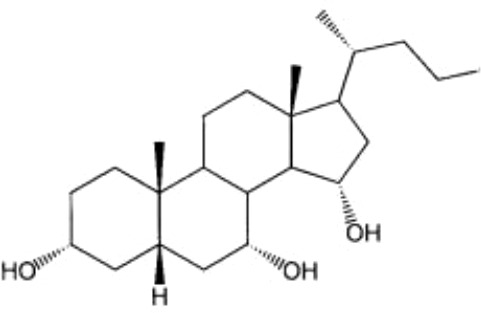
|
Bile fistula rats and bile duct ligation mice | NA | (Pellicciari et al., 2016) |
| GW4064 |
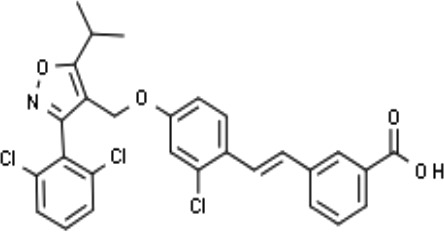
|
Short bowel resection rats associated with liver disease; endotoxin-induced hepatic inflammation; diet-induced obesity and hepatic steatosis. | NA | (Ma et al., 2013; Yao et al., 2014; Cao et al., 2019) |
| Px-102 (PX20606) and its eutomer Px-104 (GS-9674) |
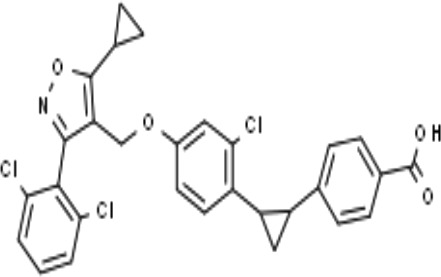
|
non-cirrhotic and cirrhotic portal hypertension; High-density lipoprotein-mediated transhepatic cholesterol efflux in mice. | Phase II for NAFLD (NCT01999101, completed). No current study | (Hambruch et al., 2012; Schwabl et al., 2017) |
| TERN-101 (LY2562175) |
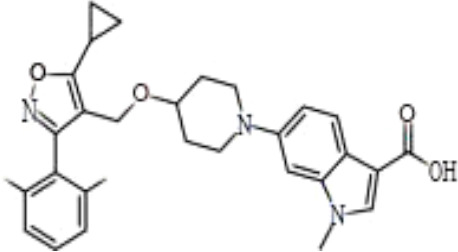
|
Dyslipidemia (LDLr-/- mice) | Phase II for NASH (NCT04328077) | (Genin et al., 2015) |
| Tropifexor (LJN452) |
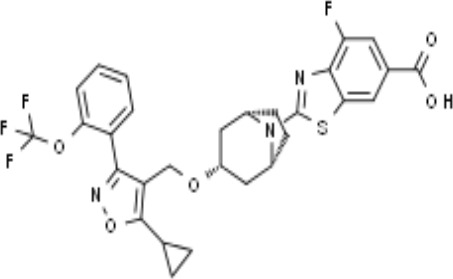
|
Amylin liver NASH model | Phase II for NAFLD and NASH (NCT03517540, NCT04147195) | (Hernandez et al., 2019) |
| WAY-362450 |
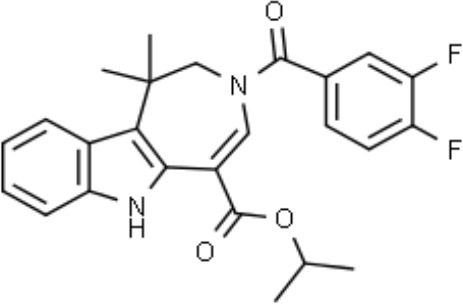
|
Maternal cholestasis; fructose-induced hepatic steatosis; murine model of alcoholic liver disease; murine model of non-alcoholic steatohepatitis. | Phase I (NCT00499629, completed; NCT00509756, terminated). No current study | (Zhang et al., 2009b; Liu et al., 2014; Wu et al., 2014; Wu et al., 2015) |
| Fexaramine |
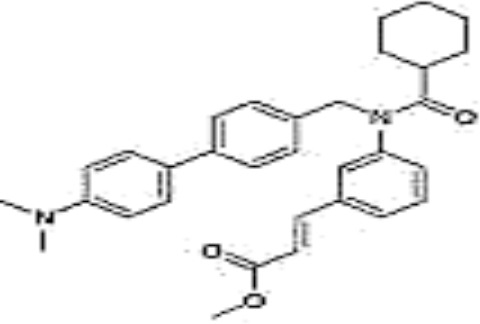
|
Alcoholic liver disease in mice; obese and diabetic mice, Lepob/ob mice; obesity and metabolic syndrome. | NA | (Fang et al., 2015; Hartmann et al., 2018; Pathak et al., 2018) |
| LMB763 |
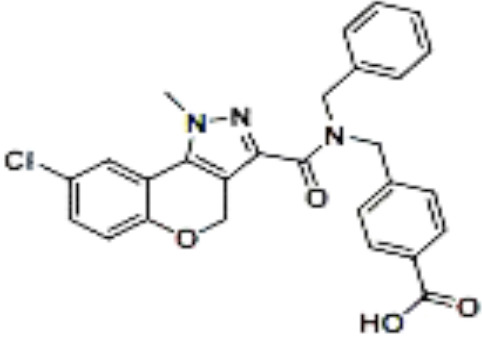
|
STAM and NASH murine mode | Phase II for Diabetic Nephropathy (NCT03804879) | (Chianelli et al., 2020) |
| EYP001 | unknown | NA | Phase II for NASH (NCT03812029); Phase II for Chronic Hepatitis B (NCT04365933) | NA |
| EDP-305 |
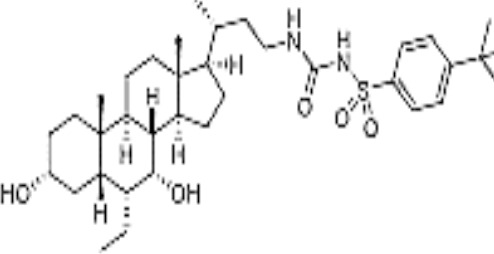
|
Biliary fibrosis and steatohepatitis mice; unilateral ureteral obstruction mice; choline-deficient, L-amino acid-defined, high-fat diet models of hepatic injury. | Phase II for Primary Biliary Cholangitis (NCT03394924); Phase II for NASH (NCT04378010) | (Erstad et al., 2018; Li S. et al., 2019; An et al., 2020) |
NA, not available; NASH, non-alcoholic steatohepatitis; NAFLD, Nonalcoholic fatty liver disease; HFD, High-fat diet.
