Abstract
The standard treatment of Sudden Sensorineural Hearing Loss is based on oral steroids. In addition, intratympanic steroid is currently used in patients who fail to respond to oral treatment. The aim of the present study was to evaluate, in patients affected by SSHL, factors that influence the response to systemic and intratympanic steroid treatment. A retrospective analysis was conducted on 149 patients, all treated with systemic steroids. Moreover, patients not responsive to systemic therapy were treated with intratympanic steroids as salvage therapy. Auditory gain was assessed through the recovery rate at the discharge and after 30 days. Statistical analysis demonstrated that patients with delayed treatment and down-sloping auditory curve presented a poor recovery. Linear and stepwise regression showed that hypertriglyceridemia and hyperglycemia were negative prognostic factors. The prognosis of SSHL is affected by hyperglycemia and hypertriglyceridemia suggesting that a microvascular dysfunction within the cochlea could impair hearing recovery. Intratympanic steroid treatment was used as salvage treatment, however in patients with poor prognostic factors or at risk for side effects, it could be used in association with systemic treatment.
Key words: sudden hearing loss, steroid therapy, intratympanic therapy, Hypertriglyceridemia, hyperglycaemia
Introduction
Sudden Sensorineural Hearing Loss (SSHL) is defined as a greater than 30 dB hearing loss over at least three contiguous frequencies, occurring suddenly or within a period of 72 hours. Although SSHL mostly afflicts people between 40 and 54 years old, it can occur at all ages. The cause of sudden hearing loss remains unknown in most patients. Several hypotheses have been proposed, among them viral infection, immune mediated involvement and vascular injury of the inner ear.1
The observation that sudden hearing loss has an acute onset, is generally unilateral and can resolve within a few hours or days, suggests that a disturbance of the cochlear microcirculation could be at the basis of this syndrome. Disturbance in cochlear blood flow due to alteration in plasma viscosity, cellular and platelet aggregability, red blood cell deformability index and endothelial function have been reported in patients affected by SSHL.2
The treatment of SSHL is controversial, however most clinicians support the use of systemic steroids together with other drugs even if the evidence of their effectiveness is poor.3 In the last years Intra-Tympanic (IT) steroids have been proposed as an effective salvage treatment in patients not responding to systemic steroids, however their superiority to systemic steroids as primary treatment is still unclear.4
Several authors have evaluated factors that influence the response to treatment. It is well known that the prognosis of SSHL declines in older patients, worse initial hearing level, longer time from onset to treatment, and presence of vertigo.5 Among haematological indices, high fibrinogen levels, high White Blood Cells (WBC) counts, homocysteine concentration, and high Low- Density Lipoprotein (LDL) correlated with poorer hearing recovery in SSHL.6-8 Our group has also reported that high levels of cholesterol are a poor prognostic factor in terms of recovery.9
The aim of the present study was to evaluate in a series of patients affected by SSHL factors that influence the response to systemic and intratympanic steroid treatment.
Materials and Methods
A retrospective analysis was conducted on 149 patients, 61 (41%) women and 88 (59%) men, hospitalized with a diagnosis of unilateral SSHL from 2013 to 2015. Data were collected after acquisition of informed consent and in accordance to Helsinki declaration. Inclusion criteria for this study were: hearing loss of >30 dB Hearing Level (HL) affecting at least three contiguous frequencies occurring in less than 72 hours; normal hearing in the contralateral ear (audiometric values in normal ranges according to age). The exclusion criteria for SSHL patients were as follows: cerebello-pontine angle pathology at MRI; an history of otologic surgery; head and/or neck trauma or barotrauma in the 10 weeks prior to SSHL diagnosis; MRI findings suggestive for congenital cochlear malformations; otitis media in the last 10 weeks; neurologic disorders predisposing to deafness; recent use of ototoxic medications. In all patients age, gender, height (cm), weight (kg), blood pressure, body mass index (BMI)(kg/m2), smoking behaviour (yes/no) were recorded at baseline and in the further observations.
In all subjects, a standardized clinical audio-vestibular investigation was carried out. It consisted of micro-otoscopy, puretone and speech audiometry, impedance audiometry, Auditory Brainstem evoked Response (ABR), Vestibular Evoked Myogenic Potential (VEMPs) and the following blood tests: Complete Blood Count (CBC), Erythrocyte Sedimentation Rate (ESR), C Reactive Protein (CRP), total cholesterol, High Density Lipoprotein (HDL), triglycerides, blood glucose and fibrinogen. In addition, in all patients Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) were calculated. Blood samples were taken before starting the treatment to rule out any related disturbance on the laboratory values.
Pure Tone Average (PTA) was calculated from the air conduction thresholds at 0.25, 0.5, 1, 2, 3, 4 and 8 kHz. Pure tone and speech audiometry were tested every 48h until hospital discharge. According to the threshold profile hearing loss was classified in three categories: upsloping (the average threshold between 0.25 and 0.50 kHz was 20 dB higher than the mean threshold of 4-8 kHz); down-sloping (the average threshold between 4 and 8 kHz was 20 dB higher than the mean threshold of 0.25-0.5 kHz), flat (less than 15 dB difference between the highest and the lowest threshold). Hearing improvement, defined in this study as hearing gain, was based on PTA obtained before and immediately after the treatment. This was calculated with the opposite ear as the healthy reference10 with the following formula:
| ??? |
This measure was assessed two times: at hospital discharge and at 30 days of follow up. In addition, four categories of recovery were defined: i) full recovery if the improvement greater than 75%; ii) good if improvement rate between 46% and 75%; iii) fair if between 20% and 45%; iv) no improvement if less than 20%. This method was used since it allows to obtain both a continuous numerical value and a categorical value for statistical analysis.
Systemic treatment was based on oral prednisone, pentoxifylline, sulphate magnesium, C vitamin and carbogen. Patients that didn’t show a significant hearing recovery with systemic therapy after on average 7 days were subjected to 3 intratympanic dexamethasone (4 mg/ml) injections performed every other day.
Statistical Analysis
To assess baseline characteristics of the sample were used means, standard deviation and ranges. Multivariate linear regressive models were run to estimate the prediction power for hearing gain of all the baseline covariates (age, sex, BMI, smoke, days before hospitalization, audiometric test, cholesterol, fibrinogen, triglycerides, ESR, PLR, NLR). Stepwise regression models, a machine learning algorithm able to exclude not significant covariates from a multivariate complex model, were used to select the best risk factor for hearing gain at discharge and after 30 days. A p value < 0.05 was considered as statistically significant with a confidence interval of 95%. In addition, non-parametric comparison tests were used to define differences among patients treated with oral steroid therapy and patients treated with oral plus intratympanic steroid therapy as salvage treatment.
Results
149 patients were included in this study. Mean age was 50.2 ± 16.9. Table 1 reports the demographic characteristics of the entire study sample. Smoking patients were 29 (25.66%). The delay between the onset of symptoms and the beginning of therapy was 6.1 ± 7.9 days. 66 patients showed a flat curve (44,3%), 54 a downsloping curve (36,24%) and 29 an upsloping curve (19,46%). In the whole group before treatment average PTA on the affected ear was 64.8 ± 26.6 dB HL and on the opposite ear was 18.6 ± 9.5 dB HL. Average PTA on the affected ear at discharge was 48,8 ± 30.5 dB HL and 41.8 ± 29.2 dB HL after 30 days. Average recovery rate was 39,6% at discharge and 53,7% after 30 days. Complete recovery occurred in 18 patients (12%). In patients undergoing intratympanic steroids injections, average PTA was 79.5 ± 25.0 dB at admission, 70,9 ± 28,5 dB at discharge and 58,4 ± 31,4 dB at 30 days. Mean recovery rate was 15.5% ± 34.1 and 35.7% ± 44.5 after 30 days. No patients presented a complete recovery.
Table 1.
Demographic and clinical characteristics of the study population at baseline.
| Parameter | Mean | SD | Min | Max | Normal Range | Outside normal range n (%) |
|---|---|---|---|---|---|---|
| Age (years) | 50.22 | 16.88 | 13 | 85 | - | - |
| BMI (kg/m2) | 25.26 | 4.42 | 15.57 | 38.78 | 18.5-24.9 | 46 (30,87%) (8 underweight; 38 overweight) |
| Fasting glucose (mg/dl) | 97.52 | 22.86 | 53 | 241 | 60-110 mg/dL | 35 (23,5%) (34: >110 mg/dL; 1:<60 mg/dL) |
| Cholesterol (mg/dl) | 182.06 | 41.56 | 96 | 290 | <200 mg/dL | 44 (29,53%) |
| HDL-C (mg/dl) | 54.73 | 14.40 | 23 | 104 | >39 (men) >45 (women) (desirable values) | 19 (12,75%) |
| Triglycerides (mg/dl) | 98.32 | 50.91 | 19 | 340 | <150 mg/dl | 17 (11,4%) |
| Fibrinogen (mg/dl) | 255.61 | 61.66 | 161 | 629 | 150-400 mg/dl | 5 (3,35%) |
| Neutrophils % | 67.36 | 11.11 | 37.80 | 91 | 45-70% | 57 (38,25%) (54: >70%; 3: <45%) |
| Lymphocyte % | 24.95 | 9.00 | 6.00 | 46.90 | 25-55% | 70 (46,97%) (70: <25%) |
| NLR | 3.48 | 2.58 | 0.98 | 15.16 | - | - |
| Platelet (103/u) | 237.54 | 62.13 | 57 | 402 | 150-450 | 11 (7,38%) (11: <150) |
| PLR | 11.68 | 8.05 | 3.37 | 61.66 | - | 11 (7,38%) |
| ESR (mm/h) | 12.52 | 9.83 | 2.00 | 46 | <20 (men) <30 (women) | (11: <150) |
BMI: Body Mass Index; HDL: High-Density Lipoprotein; NLR: Neutrophil-Lymphocyte Ratio; PLR: Platelet-Lymphocyte Ratio; ESR: Erythrocyte Sedimentation Rate; Mean: Arithmetic average; SD: Standard Deviation; Min: Minimum value; Max: Maximum value.
In Table 2 the results of a linear regression analysis evaluating prognostic factors in terms of hearing gain at discharge are reported. Longer delay between the occurrence of SNHL and the treatment (p = 0.05) and a down sloping audiometric curve at admission (p= 0.001) predicted a lower hearing gain. This model was fully adjusted for all the covariates of the baseline Table 1 (Age, BMI, Fasting glucose, Cholesterol, HDL-C, Triglycerides, Fibrinogen, Neutrophils, Lymphocytes, NLR, Platelet, PLR, ESR).
Stepwise regressive models for the hearing gain at discharge are shown in Table 3A. The best prediction power for a reduced hearing gain at discharge were time interval between hospitalization and treatment, down-sloping curve and triglycerides values. In the same model, male sex was a protective factor (Table 3A). Stepwise regressive models for the hearing gain at follow up (30 days after discharge) are reported in Table 3B and show that lower gain was associated only with higher blood glucose concentration (Table 3B).
In Figure 1 the average recovery rate at discharge (%)in relationship to the shape of audiometric curve is reported.
The comparison between patients treated only with systemic therapy and those receiving IT steroids showed that patients that received salvage IT steroids were significantly older (p=0.00670) and presented a poorer hearing threshold at hospitalization (p<0.0000). No other differences were identified (Table 4).
Table 2.
Linear regression analysis correlating recovery rate at discharge (%) as dependent variable vs age, sex, PTA, days before hospitalization, audiometric curve.
| Covariates | Estimate | P value | Conf. Low | Conf. High |
|---|---|---|---|---|
| Age | -0.12745 | 0.53723 | -0.53480 | 0.27991 |
| Sex M | 12.99419 | 0.06467 | 0.80105 | 26.78942 |
| PTA (t0) | 0.03609 | 0.82860 | -0.29281 | 0.36498 |
| Days before hospitalization | -0.80608 | 0.05945 | -1.64471 | 0.03256 |
| Downsloping curve | -24.81227 | 0.00102 | -39.43701 | -10.18754 |
| Upsloping curve | -9.68262 | 0.29293 | -27.81511 | 8.44988 |
Table 3.
(A) Stepwise regression analysis correlating recovery rate at first discharge as dependent variable with all the covariates of the Table 1. (B) Stepwise regression analysis correlating recovery rate after 30 days as dependent variable with all the covariates of Table 1.
| A. Stepwise regression analysis using recovery rate at discharge as dependent variable | B. Stepwise regression analysis using recovery rate after 30 days as dependent variable | ||||
|---|---|---|---|---|---|
| Covariates | Estimate | P value | Covariates | Estimate | P value |
| Sex M | 23.66940 | 0.00417 | Downsloping curve | -20.60410 | 0.10900 |
| Downsloping curve | -26.21127 | 0.00506 | Upsloping curve | 7.38494 | 0.64880 |
| Days before hospitalization | -0.87708 | 0.06735 | Neutrophils | -1.30304 | 0.14271 |
| Triglycerides | -0.17339 | 0.05054 | PLR | 1.57166 | 0.14885 |
| Upsloping curve | -3.58020 | 0.73185 | Cholesterol | 0.20049 | 0.17282 |
| BMI | 1.62598 | 0.08087 | Blood glucose | -0.38730 | 0.04986 |
Table 4.
Comparison among patients treated with oral steroid therapy and patients treated with oral + intratympanic steroid therapy as salvage treatment.
| Variables | Variables | Oral + intratympanic steroid therapy | P value |
|---|---|---|---|
| Age | Age | 54.19 ± 15.18 | 0.00670 |
| Sex F | Sex F | 42.03(29) | 1 |
| PTA (t0) | PTA (t0) | 79.51 ± 24.96 | 0.000 |
| Smoke (no smokers) | Smoke (no smokers) | 77.27(34) | 0.7264568 |
| PLR | PLR | 11.22 ± 6.65 | 0.51347 |
| NLR | NLR | 3.6 ± 2.59 | 0.62897 |
| Fibrinogen | Fibrinogen | 255.44 ± 69.33 | 0.97742 |
| VES | VES | 13.9 ± 9.31 | 0.14987 |
| Cholesterol | Cholesterol | 187.4 ± 41.19 | 0.16521 |
| BMI | BMI | 26.05 ± 4.2 | 0.06941 |
Discussion
The results of the present study showed that time between the onset of symptoms and the beginning of treatment, and down-sloping audiometric curve are negative prognostic factors in patients affected by SSHL. In addition, stepwise regression analysis demonstrated that high levels of triglycerides and fasting glucose were correlated with poorer recovery. The relation between a prompt treatment and hearing recovery is well known and has been reported, among others, by Byl11 in 1984.Wei et al.12 reported that best hearing results are achieved if the treatment is started within 9 days, while other authors showed that treatment of hearing loss during the first week after the onset of symptoms led to better results, independently from the age of the patient.13 The degree of hearing loss and the audiogram profile are well known prognostic factors, severe to profound HL and down-sloping thresholds in fact are associated with a worse chance of recovery.14
The role of glucose and lipid concentration, as both etiologic and prognostic factors for recovery, has been evaluated more recently.Fasano et al.15 reported that higher levels of blood glucose and glycated haemoglobin (HbA1C) were found in SSHL patients compared to controls. It was hypothesized the hyperglycaemia, insulin resistance and fatty acid excessive production, that lead to systemic oxidative stress, inflammatory response, and advanced glycation end-product production, contribute to atherosclerosis onset and microvascular dysfunction with subsequent damage and apoptosis of endothelial cells. Similar findings were reported by Lin et al.16 that showed how diabetes mellitus was significantly associated with an increased risk of developing SSHL, especially in patients with associated coronary heart disease or diabetic retinopathy.
The role of hyperglycaemia as a negative prognostic factor for recovery has also been reported by other authors.17,18 Both microvascular dysfunction and the neuropathy secondary to hyperglycaemic status have been associated with poorer recovery. Hyperglycaemia as a negative prognostic factor needs to be further investigated since other authors19 have not demonstrated a correlation of glucose concentration with hearing recovery.
The results of the present study confirm and highlight the role of lipids as a prognostic factor in SSHL. Dyslipidaemia (hypercholesterolemia and/or triglyceridemia) in target organs, such as the cochlea, can disrupt blood flow through the processes of plaque formation, vascular remodelling and vascular luminal obstruction, inducing endothelial dysfunction and increasing the lipid accumulation on the intima of vessel walls.17 The negative prognostic value of total cholesterol concentrations and of high LDL/HDL ratio have already been reported.6,7,20 Lin et al.21 provided evidence that comorbid diabetes or hypercholesterolemia may indicate a smaller probability of improvement for patients with SSHL, moreover a recent work by Jung et al. demonstrated that the rate of recovery from SSHL was lower among patients with metabolic syndrome in particular patients with 4 or more diagnostic criteria (Hyperglycemia or type 2 diabetes, hypertriglyceridemia, hypertension, obesity, HDL reduction).17
IT steroids were firstly proposed as salvage treatment in SSHL patients in 200422 and a recent meta-analysis23 demonstrated their effectiveness. As reported by other authors,12,24 the comparison of patients that underwent IT steroids with those treated with systemic treatment showed that higher age and worse hearing threshold at admission increased the chance of having IT steroids. Recently the non-inferiority of IT steroids to systemic steroids as first line treatment in ISSHL patients has been reported by two meta-analysis4,25 and therefore IT steroids have been proposed as first line treatment in patients at risk for systemic steroids side effects. In addition, Ahmadzai et al.26 have shown, using treatment network based on the literature, that systemic plus IT steroids are associated with the largest differences in PTA change compared to other treatment options.
Figure 1.
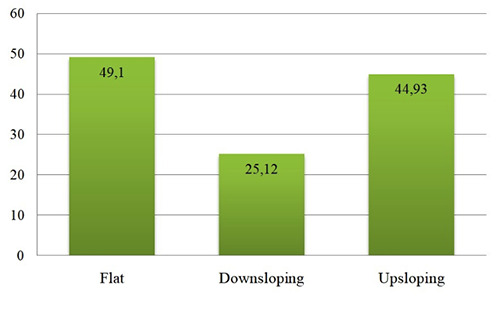
Average recovery rate at discharge (%) is reported in relation to the shape of audiogram.
Conclusions
Our study confirms that a longer interval between onset of hearing loss and treatment and down-sloping audiometric curve are associated with worse hearing recovery. In addition, stepwise regression analysis showed that the prognosis was also affected by hyperglycaemia and hypertriglyceridemia suggesting that a microvascular dysfunction within the cochlea could impair hearing outcomes. IT steroid treatment was used as salvage treatment, however in patients with poor prognostic factors or at risk for systemic steroids side effects, it could be used in association with systemic treatment.
References
- 1.Chau JK, Lin JR, Atashband S, et al. Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. Laryngoscope 2010;120:1011–21. [DOI] [PubMed] [Google Scholar]
- 2.Quaranta N, De Ceglie V, D'Elia A. Endothelial Dysfunction in Idiopathic Sudden Sensorineural Hearing Loss: A Review. Audiol Res 2016;6:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plontke SK. Diagnostics and therapy of sudden hearing loss. GMS Curr Top Otorhinolaryngol Head Neck Surg 2018;16:Doc05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai D, Zhao F, Jalal N, et al. Intratympanic glucocorticosteroid therapy for idiopathic sudden hearing loss: Meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017;96:e8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisenman D, Arts HA. Effectiveness of treatment for sudden sensorineural hearing loss. Arch Otolaryngol Head Neck Surg 2000;126:1161–4. [DOI] [PubMed] [Google Scholar]
- 6.Cao Z, Li Z, Xiang H, et al. Prognostic role of haematological indices in sudden sensorineural hearing loss: Review and meta-analysis. Clin Chim Acta 2018;483:104-11. [DOI] [PubMed] [Google Scholar]
- 7.Lee EJ, Cho YJ, Yoon YJ. Methylenetetrahydrofolate reductase C677T gene mutation as risk factor for sudden sensorineural hearing loss: association with plasma homocysteine, folate and cholesterol concentrations. J Laryngol Otol 2010;124: 1268–73. [DOI] [PubMed] [Google Scholar]
- 8.Capaccio P, Pignataro L, Gaini LM, et al. Unbalanced oxidative status in idiopathic sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol 2012;269:449–53. [DOI] [PubMed] [Google Scholar]
- 9.Quaranta N, Squeo V, Sangineto M, et al. High total cholesterol in peripheral blood correlates with poorer hearing recovery in idiopathic sudden sensorineural hearing loss. PLoS One 2015;10:e0133300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkins S. A., Mattox D. E., Lyles A. Evaluation of a “shotgun” regimen for sudden hearing loss. Otolaryngol Head Neck Surg 1987;97:474-80. [DOI] [PubMed] [Google Scholar]
- 11.Byl FM. Sudden hearing loss: eight years' experience and suggested prognostic table. Laryngoscope 1984;94:647-61. [PubMed] [Google Scholar]
- 12.Wei X, He J. [Analysis of prognostic factors for sudden sensorineural hearing loss.] Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2011;25:599-601. [Article in Chinese]. [PubMed] [Google Scholar]
- 13.Enache R, Sarafoleanu C. Prognostic factors in sudden hearing loss. J Med Life 2008;1:343-7. [PMC free article] [PubMed] [Google Scholar]
- 14.Cvorović L, Deric D, Probst R, et al. Prognostic model for predicting hearing recovery in idiopathic sudden sensorineural hearing loss. Otol Neurotol 2008;29:464-9. [DOI] [PubMed] [Google Scholar]
- 15.Fasano T, Pertinhez TA, Tribi L, et al. Laboratory assessment of sudden sensorineural hearing loss: A case-control study. Laryngoscope 2017;127:2375-81. [DOI] [PubMed] [Google Scholar]
- 16.Lin SW, Lin YS, Weng SF, et al. Risk of developing sudden sensorineural hearing loss in diabetic patients: a populationbased cohort study. Otol Neurotol 2012;33:1482–8. [DOI] [PubMed] [Google Scholar]
- 17.Jung SY, Shim HS, Hah YM, et al. Association of Metabolic Syndrome With Sudden Sensorineural Hearing Loss. JAMA Otolaryngol Head Neck Surg 2018;144:308-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryu OH, Choi MG, Park CH, et al. Hyperglycemia as a potential prognostic factor of idiopathic sudden sensorineural hearing loss. Otolaryngol Head Neck Surg 2014;150:853–8. [DOI] [PubMed] [Google Scholar]
- 19.Hiraumi H, Yamamoto N, Sakamoto T, et al. Multivariateanalysis of hearing outcomes in patients with idiopathic sudden sensorineural hearing loss. Acta Otolaryngol Suppl 2010;563:24-8. [DOI] [PubMed] [Google Scholar]
- 20.Lin HC, Wang CH, Chou YC, et al. The correlation between lipoprotein ratios and hearing outcome in idiopathic sudden sensorineural hearing loss patients. Clin Otolaryngol 2015;40:355-62. [DOI] [PubMed] [Google Scholar]
- 21.Lin CF, Lee KJ, Yu SS, et al. Effect of comorbid diabetes and hypercholesterolemia on the prognosis of idiopathic sudden sensorineural hearing loss. Laryngoscope 2016;126:142-9. [DOI] [PubMed] [Google Scholar]
- 22.Ho HG, Lin HC, Shu MT, et al. Effectiveness of intratympanic dexamethasone injection in sudden-deafness patients as salvage treatment. Laryngoscope 2004;114:1184-9. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Feng G, Wang H, et al. Intratympanic steroid therapy as a salvage treatment for sudden sensorineural hearing loss after failure of conventional therapy: a meta-analysis of randomized, controlled trials. Clin Ther 2015;37:178-87 [DOI] [PubMed] [Google Scholar]
- 24.Kang WS, Yang CJ, Shim M, et al. Prognostic Factors for Recovery from Sudden Sensorineural Hearing Loss: A Retrospective Study. J Audiol Otol 2017;21:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao D, Tong B, Wang Q, et al. A comparison of effects of systemic and intratympanic steroid therapies for sudden sensorineural hearing loss: A meta-analysis. J Otol 2016;11:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmadzai N, Kilty S, Cheng W, et al. A systematic review and network meta-analysis of existing pharmacologic therapies in patients with idiopathic sudden sensorineural hearing loss. PLoS One 2019;14:e0221713. [DOI] [PMC free article] [PubMed] [Google Scholar]


