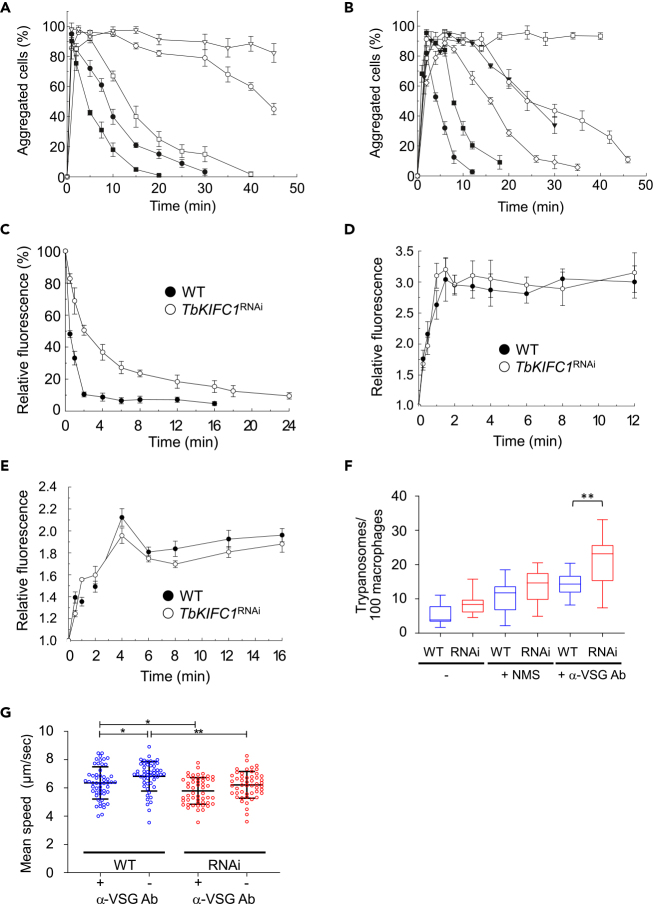
Figure 4.
Role of TbKIFC1 in Surface Trafficking of VSG/Antibody Complexes
(A) Disaggregation of WT or clone 1 TbKIFC1RNAi cells following addition of anti-VSG IgGs as follows: WT: ■, 1 μg/mL; ●, 2 μg/mL; TbKIFC1RNAi cells: □, 1 μg/mL; ◯, 2 μg/mL; ▽, 3 μg/mL. Data are represented as mean ± SD; n = 3.
(B) Disaggregation of WT and TbKIFC1RNAi cells following addition of purified anti-VSG IgMs as follows: WT: ▼, 40 μg/mL; ■, 16 μg/mL; ●, 8 μg/mL. TbKIFC1RNAi cells: □, 16 μg/mL; ◯, 8 μg/m; ◇, 4 μg/mL. Data are represented as mean ± SD; n = 3.
(C) Clearance of surface-bound antibodies as determined by flow cytometry of WT and clone 1 TbKIFC1RNAi cells incubated with anti-VSG immune serum. The data are expressed as percent median fluorescence intensity detected relative to time zero. Data are represented as mean ± SD; n = 3.
(D) Internalization of surface-biotinylated VSG. After incubation at 37°C for various times, internalization of surface-biotinylated WT in clone 1 TbKIFC1RNAi cells was determined by flow cytometry. The data are expressed as percent median fluorescence intensity detected relative to time zero. Data are represented as mean ± SD; n = 3.
(E) Return of internal biotinylated VSG to the surface. After incubation at 37°C for 15 min, membrane traffic of surface-biotinylated WT and TbKIFC1RNAi cells (clone 1) was cold-stopped and the remaining surface biotin was removed with reduced glutathione. The appearance of surface biotin was determined by flow cytometry after incubation for various times at 37°C. The data are expressed as in (D). Data are represented as mean ± SD; n = 3.
(F) Binding and uptake of WT and TbKIFC1RNAi parasites by murine RAW264.7 macrophages, after parasite incubation or not for 30 min with either 10% normal mouse serum (NMS) or anti-MITat 1.1 VSG antibodies. Data are represented as mean ± SD; n = 3. One-way ANOVA, Sidak's multiple comparison test ∗∗p < 0.01.
(G) Mobility of WT and TbKIFC1RNAi trypanosomes after incubation or not with anti-MITat 1.1 VSG antibodies. The data are from a representative experiment. Data are represented as mean ± SD; n = 3. ANOVA/Dunn's multiple comparison test ∗p < 0.01; ∗∗p < 0.05