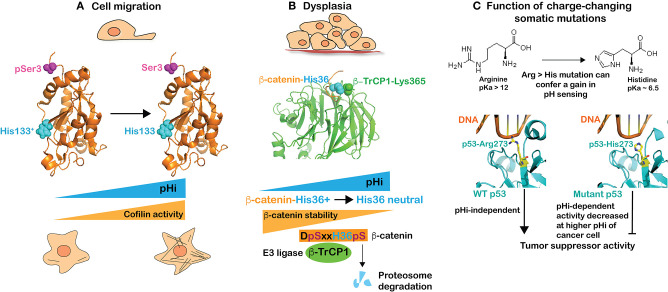
Figure 1.
The higher pHi of cancer cells enables many behaviors, including directional migration and tumorigenesis as well as the tumorigenic functions of proteins with charge-changing arginine to histidine mutations. (A) Cell migration is in part dependent on increased activity of cofilin with increased pHi. Cofilin is a coincidence-regulated pH sensor that is activated by deprotonation of His133 (cyan) and dephosphorylation of Ser3 (magenta) for actin polymerization enabling cell migration. (B) Dysplasia is associated with increased pHi, which decreases β-catenin stability. β-catenin is a coincidence-regulated pH sensor with deprotonation of His36 (cyan) and phosphorylation of Ser33/37 by GSK3β enabling binding to the E3 ligase β-TrCP1 for targeting to the proteasome for degradation. Crystal structure data show that β-catenin-His36 is in close proximity to β-TrCP1-Lys365, which suggest that binding would be electrostatically unfavorable with a protonated His36 at lower pHi. (C) Charge changing somatic mutations can confer pH-regulated protein activity. Structure of wild-type p53 (left) and mutant p53-R273H (right) in complex with DNA indicating an electrostatic interaction of Arg273 with the negatively charged phosphate-backbone of DNA that could be partially enabled by protonated, but not neutral, His273.