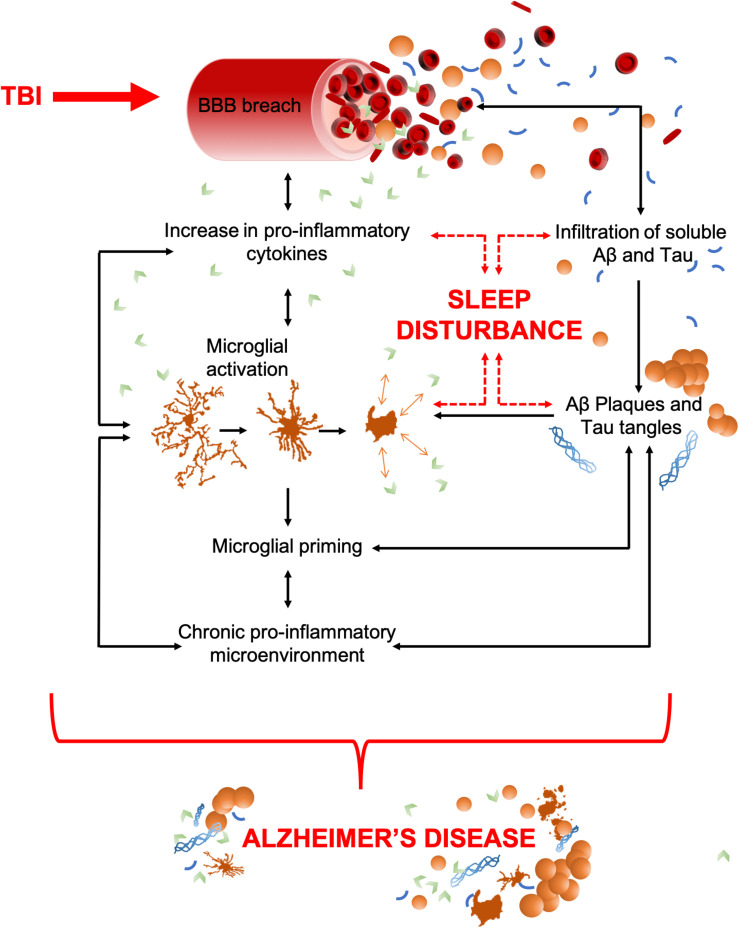
FIGURE 1.
TBI to AD, an inter-disease trajectory. TBI disrupts the blood brain barrier (BBB) upon insult which results in an infiltration of peripheral pro-inflammatory cytokines and any soluble pools of amyloid-β (Aβ) and Tau. Together, these can precipitate AD. As some pro-inflammatory cytokines have dual (opposing) roles as sleep regulatory substances, their increase can also lead to sleep disturbances, a characteristic that commonly precedes the cognitive decline in AD. Pro-inflammatory cytokines upregulate the activation of microglia, which act as a positive feedback mechanism, resulting in increased pro-inflammatory cytokine production and an increased breach of the BBB. Unregulated cytokine release also sustains microglial activation and priming which results in a chronic pro-inflammatory microenvironment. This includes astrocytosis, hypoxia, reactive oxygen species (ROS), elevated cytokine levels, and microglial activation. The movement of amyloid-β (Aβ) and Tau through the breach in the BBB could potentially seed protein oligomerization and aggregation, thereby acting as possible drivers of central plaque and tangle pathology. Such aggregates in the brain further contribute to microglial activation, the pro-inflammatory microenvironment, and neuronal apoptosis. Together, these contribute to cognitive dysfunction and brain atrophy, the key pathological features of AD. Both brain atrophy and neuronal death help to sustain the pro-inflammatory microenvironment creating a self-perpetuating feedback loop.