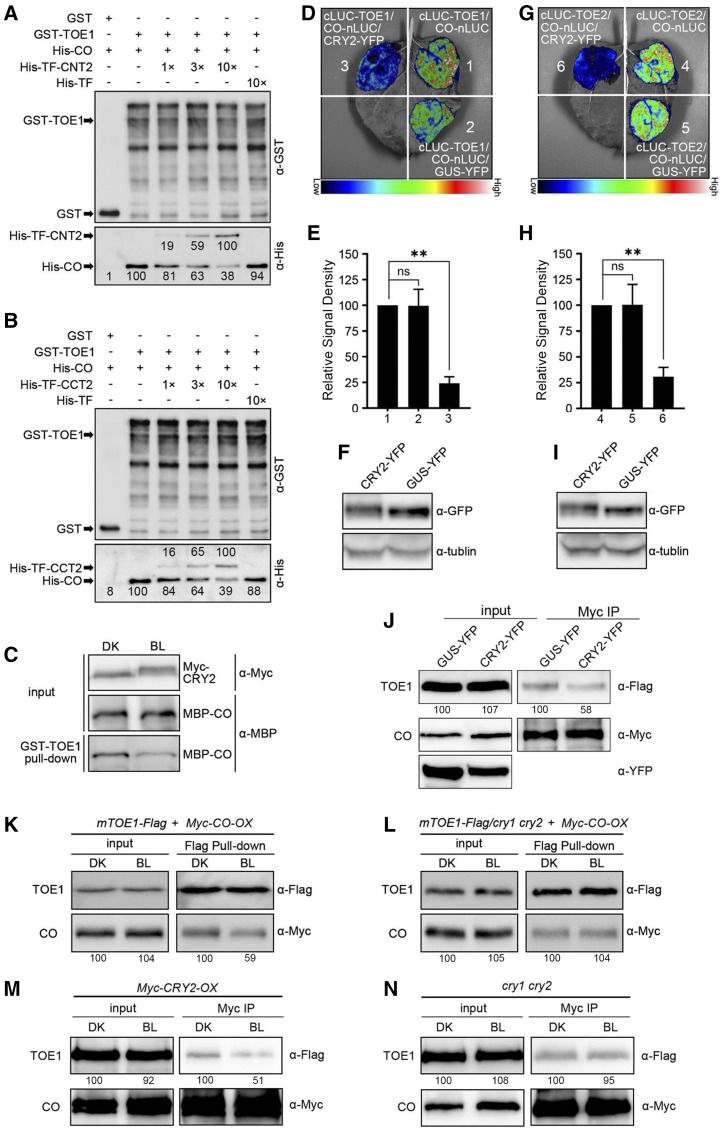
Figure 6.
CRY2 represses the interactions of CO with TOE1 and TOE2 in vitro and in vivo. A and B, GST pull-down assays showing repression of the association of CO with TOE1 by CNT2 (A) and CCT2 (B). GST-TOE1 served as bait. His-TF served as negative control. The relative band intensity was normalized to the indicated sample for each image and shown near each band. C, Semi-in vivo pull-down assays showing repression of the association of CO with TOE1 by CRY2 in a BL-specific manner. GST-TOE1 served as bait. Myc-CRY2 protein extracted from Myc-CRY2-OX seedlings that were grown for 5 d in white light and then transferred to the dark for 3 d. Seedlings were then initially treated with 100 μm of MG132 for 4 h and finally transferred into DK or exposed to BL (20 μmol m−2 s−1) and MBP-CO served as preys. The prey proteins pulled down by GST-TOE1 were detected with anti-Myc antibody and anti-MBP antibody. D to F, Split-LUC complementation imaging assays indicating CRY2 represses the interaction of CO with TOE1 in N. benthamiana cells. Lower luminescence intensity was observed after CRY2-YFP cotransformed into N. benthamiana leaf epidermal cells compared with the control plants, which were cotransformed GUS-YFP (D). E, The luminescence intensity was quantitatively analyzed. Data are represented as the mean of biological triplicates ± sd (n = 4). Asterisks indicated significant difference by Student’s t test (**P < 0.01). F, The expression of CRY2-YFP and GUS-YFP was detected by anti-GFP antibody. G to I, Split-LUC complementation imaging assays indicating CRY2 represses the interaction of CO with TOE2 in N. benthamiana cells. Lower luminescence intensity was observed after CRY2-YFP cotransformed into N. benthamiana leaf epidermal cells compared with the control plants that were cotransformed GUS-YFP (G). H, The luminescence intensity was quantitatively analyzed. Data are represented as the mean of biological triplicates ± sd (n = 4). Asterisks indicated significant difference by Student’s t test (**P < 0.01). I, The expression of CRY2-YFP and GUS-YFP was detected by anti-GFP antibody. J, Co-IP assays showing that CRY2 represses the interaction of CO with TOE1 in N. benthamiana. TOE1-Flag, Myc-CO, and GUS-YFP or CRY2-YFP were coexpressed in N. benthamiana leaves. Myc-CO served as bait, and TOE1-Flag served as prey. GUS-YFP and CRY2-YFP were detected with anti-GFP antibody. The assays were repeated three times with similar results. K to l, Semi in vivo pull-down assays showing CRY2 inhibition of the interaction of TOE1 with CO (K). TOE1-Flag protein extracts served as bait, which were prepared from mTOE1-Flag and mTOE1-Flag/cry1 cry2 seedlings grown for 5 d in white light and then transferred to the dark for 3 d, treated with 100 μm of MG132 for 4 h, and adapted to DK or exposed to BL (30 μmol m−2 s−1). Myc-CO protein extracts from BL-adapted Myc-CO-OX seedlings served as prey. The assays were repeated three times with similar results. M and N, Co-IP assays showing CRY2 inhibition of the interaction of TOE1 with CO (M) in Arabidopsis protoplasts. TOE1-Flag and Myc-CO were coexpressed in Myc-CRY2-OX and cry1 cry2 protoplasts. The transformed protoplasts were exposed to BL (30 μmol m−2 s−1) for 16 h and then treated with 100 μm of MG132 for 1 h, and adapted to DK or exposed to BL for 2 h. TOE1-Flag served as prey and Myc-CO served as bait. The assays were repeated twice with similar results. The relative band intensity was normalized to the sample adapted to DK for each image and shown below each lane (J–N).