Figure 4.
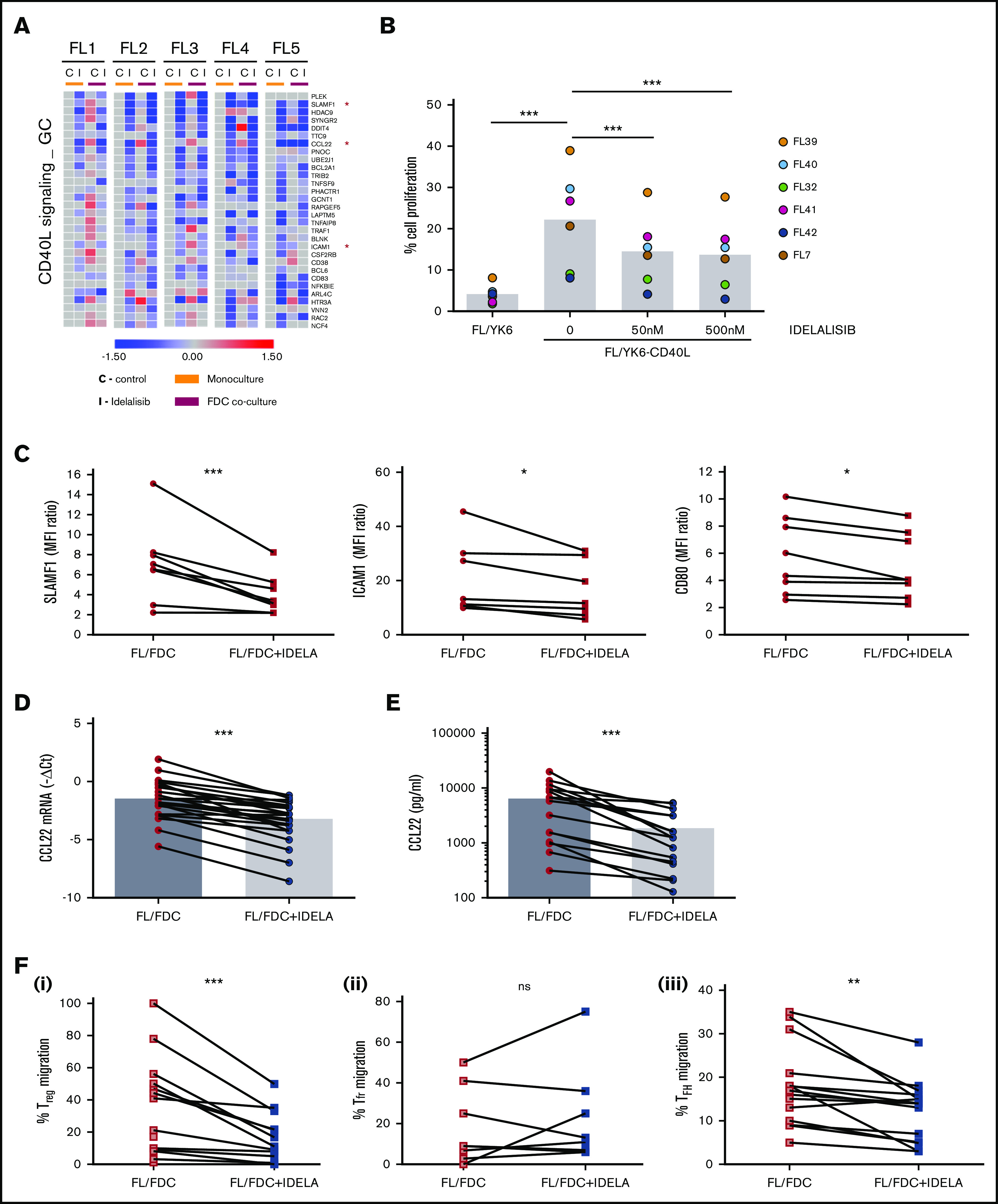
Idelalisib interferes with FL–T cells cross talk through CD40/CD40L and affects Treg and TFH recruitment through CCL22 downregulation. (A) FL cells (n = 5) were cultured for 48 hours with idelalisib (500 nM), and B cells were purified and subjected to GEP. Gene sets regulated by idelalisib (IDELA) in the presence or absence of FDC coculture were identified by GSEA using custom genes set (http://lymphochip.nih.gov/signaturedb/index.html). A heatmap of the leading edge corresponding to the CD40L_signaling_GC gene set is shown and represents the relative gene expression of FL cells cultured with and without IDELA compared with the untreated control. (B) FL cells (n = 7) were labeled with carboxyfluorescein succinimidyl ester (CFSE; 0.5μM) and cocultured for 5 days with and without 500 nM idelalisib on pre-established layers of FDCs engineered or not for CD40L expression (YK6 and YK6-CD40L). The percentage of viable CD19+ cells with low CFSE fluorescence was used as a read-out of proliferation. (C) SLAMF1, ICAM1, and CD80 membrane expression was evaluated by flow cytometry in FL-FDC cocultures (n = 8) with and without IDELA (500 nM, 48 hours). FL-FDC coculture supernatants with and without IDELA were used to assess CCL22 gene expression by real-time polymerase chain reaction (n = 26) using GUSB, ACTB, and B2M as houskeeping genes (D), CCL22 protein expression by ELISA (n = 16) (E), and migration of Treg cells (CD4+CD25+FoxP3+; n = 14) obtained from PBMCs of healthy donors (Fi) or migration of Tfr (CD4+CXCR5+FoxP3+; n = 9) (Fii) and TFH cells (CD4+CXCR5+CD25−; n = 14) (Fiii) obtained from normal tonsils.
