Figure 3.
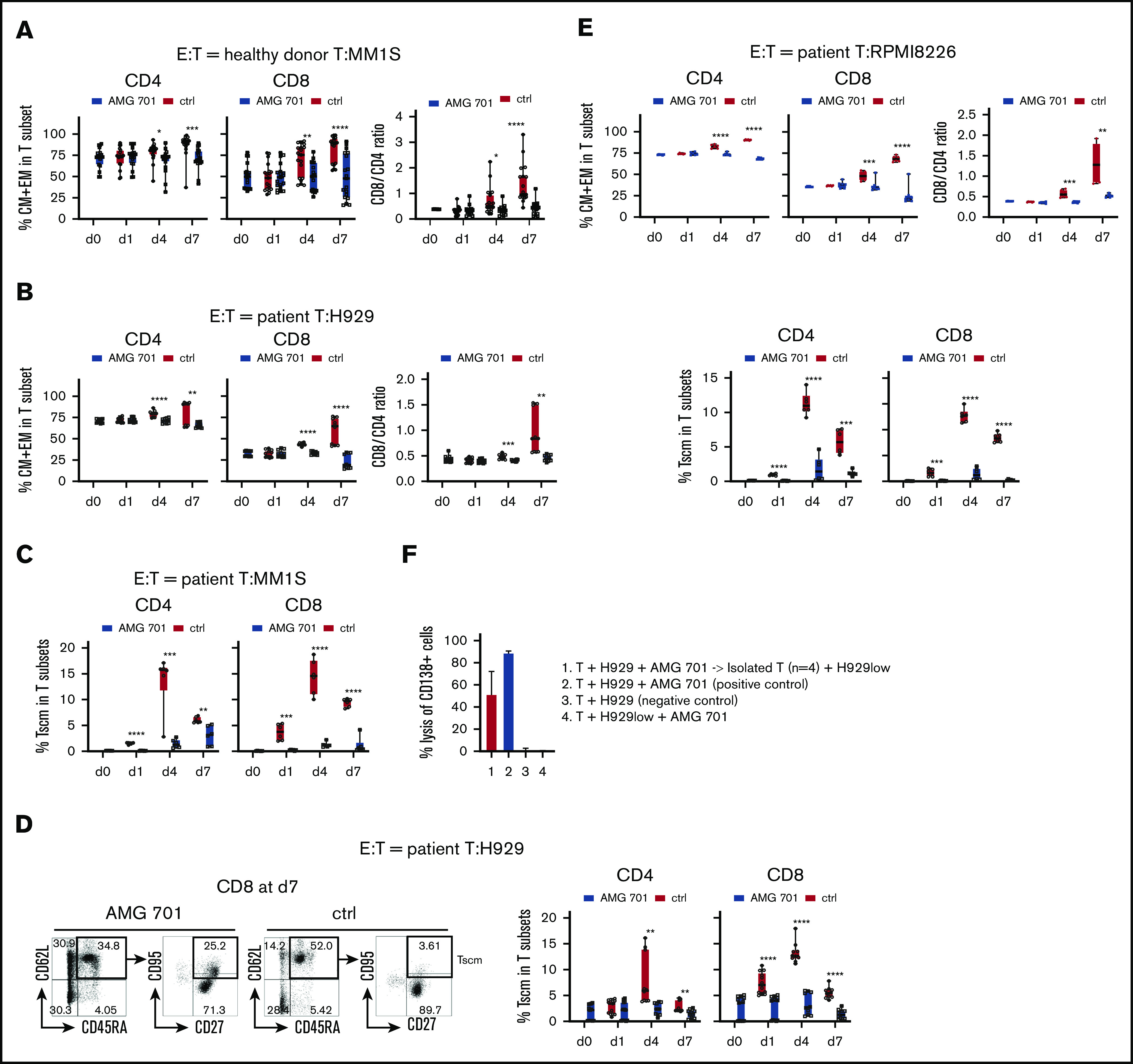
AMG 701 induced T-cell differentiation including scm phenotype, associated with further increases in CD8/CD4 ratios. T cells from donors (A; n = 13) or MM patients (B; n = 5; E, n = 3) were incubated with indicated target MM cells (A, MM1S; B, H929; E, RPMI8226) (E:T = 10:1) in AMG 701 (93.4 pM) or control (ctrl) groups on day 0 (d0). Quantitative FC analysis was used to determine the percentage of differentiated T-cell subtypes: naive (CD45RA+CD62L+), CM (CD45RA−CD62L+), EM (CD45RA−CD62L−), and TE (CD45RA+CD62L−). (A-B, E top panel) Shown are the percentage of CM and EM (means ± SDs) in CD4 and CD8 T subsets (left panel), as well as CD8/CD4 ratios (right panel), at indicated time periods. (C-D) T cells from additional patient samples (C, n = 3; D, n = 5) were coincubated with MM1S (C) or H929 (D) target cells as in panels A and B. (C-D, E bottom panel) FC analysis was used to measure the percentage of Tscm (CD27+CD95+CD45RA+CD62L+) in CD4 and CD8 T subsets. (D) Representative dot plots are shown (left panel) demonstrating gating strategies to define Tscm in CD8 T cells on day 7 in AMG 701 (left) and ctrl (right) groups. (F) T cells from patients (n = 4) were purified after 4-day coculture with H929 target cells (E:T=10:1) in the presence of AMG 701 (93.4 pM). The isolated T cells were then incubated for 24 hours with BCMA-knockdown H929low cells. BCMA ABC was 12 888 vs 273 in the parental H929 vs H929low cells. Shown are the % lysis (means ± SDs) of CD138+ MM target cells (1 and 4, H929low; 2-3, H929) following 24-hour incubation at indicated conditions. Four independent experiments were done at each condition. All data are shown as means ± SDs. *P < .05; **P < .005; ***P < .001; ****P < .0001.
