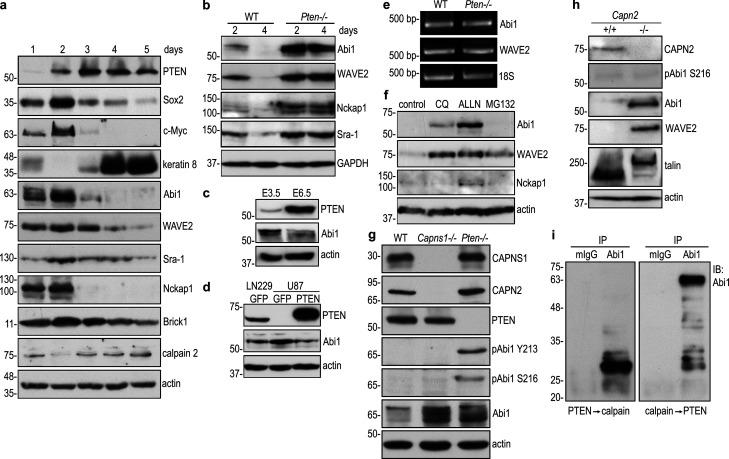
Figure 3.
PTEN-mediated dephosphorylation of Abi1 leads to Abi1 degradation by calpains. (a) Wild-type EBs were cultured for 1–5 d and analyzed by immunoblotting. (b) 2- and 4-d wild-type and Pten−/− EBs were analyzed by immunoblotting. (c) E3.5 and E6.5 embryos were isolated from timed wild-type pregnant mice and analyzed by immunoblotting. (d) The protein level of Abi1 was higher in PTEN-negative (U87) human glioblastoma cells when compared with those that were PTEN positive (LN229). Subsequent restoration of PTEN expression in U87 cells down-regulated Abi1. (e) RT-PCR analysis for Abi1 and WAVE2 in 4-d wild-type and Pten−/− EBs. (f) 4-d EBs were treated with the lysosomal inhibitor chloroquine (CQ), the calpain inhibitor ALLN, or the proteasome inhibitor MG-132 for 16 h. Immunoblotting was performed to detect the WAVE complex subunits. (g) Wild-type, calpain small subunit 1 (Capns1)–, and Pten-null (−/−) EBs were cultured for 5 d and analyzed by immunoblotting. (h) Immunoblot analysis of 5-d wild-type and calpain 2 (Capn2)-null EBs showed that Abi1 and WAVE2 were significantly increased in Capn2−/− EBs. Intact talin was detected in Capn2−/− EBs, but not in wild-type EBs. Cleaved talin (the rod domain) was mainly detected in wild-type EBs. (i) Abi1 was immunoprecipitated from 5-d Pten−/− EBs with anti-Abi1 monoclonal antibody. Preimmune mouse IgG (mIgG) served as a control. The immunoprecipitates were incubated with active PTEN for 30 min, washed with PBS, and then treated with active porcine calpain 1 for an additional 30 min. Enzymatic digestion in the reverse order of above was also performed with mouse IgG immunoprecipitates as control. Digestion products were analyzed by immunoblotting using rabbit anti-Abi1 antibody.