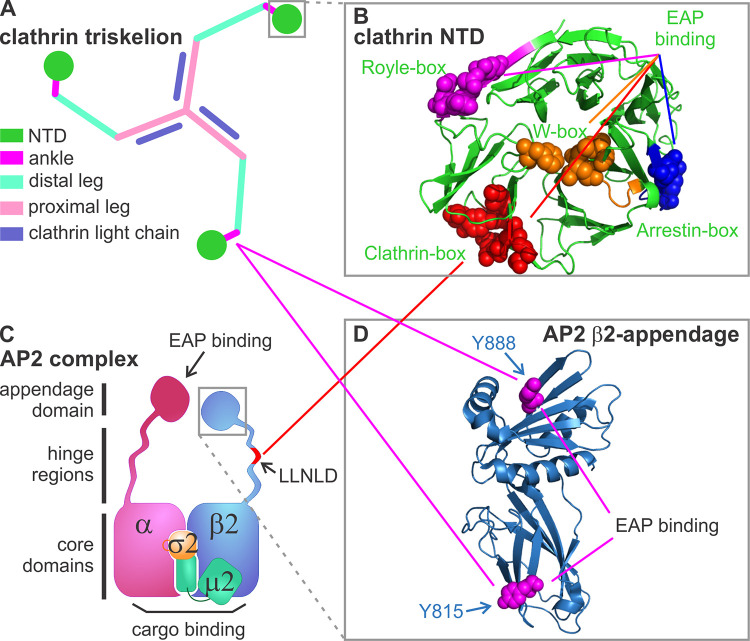
Figure 1.
Known AP2–clathrin interactions. (A) Diagram of a clathrin triskelion that is composed of three heavy chains and three light chains. Each heavy chain contains an NTD (dark green), which connects to an ankle region (magenta), a distal leg (light green), and a proximal leg (pink) where light chain (purple) binds. (B) Structure of the NTD, which contains four known binding sites defined by key residues (shown by spheres): the clathrin-box (red), W-box (orange), arrestin-box (blue), and Royle-box (magenta; Lemmon and Traub, 2012; Willox and Royle, 2012; modified from PDB accession no. 1BPO). (C) Diagram of the heterotetrameric AP2 complex composed of α, β2, μ2, and σ2 subunits. The clathrin binding sites are located on the hinge region (i.e., the LLNLD clathrin-box motif) of the β2 subunit (shown in red). (D) Structure of the β2-appendage domain (modified from PDB accession no. 1E42), which contains two additional clathrin binding sites bearing key residues, Y815 and Y888 (shown by spheres). Solid red and magenta lines indicate interactions mediated by the clathrin-box and β-appendage domains, respectively.