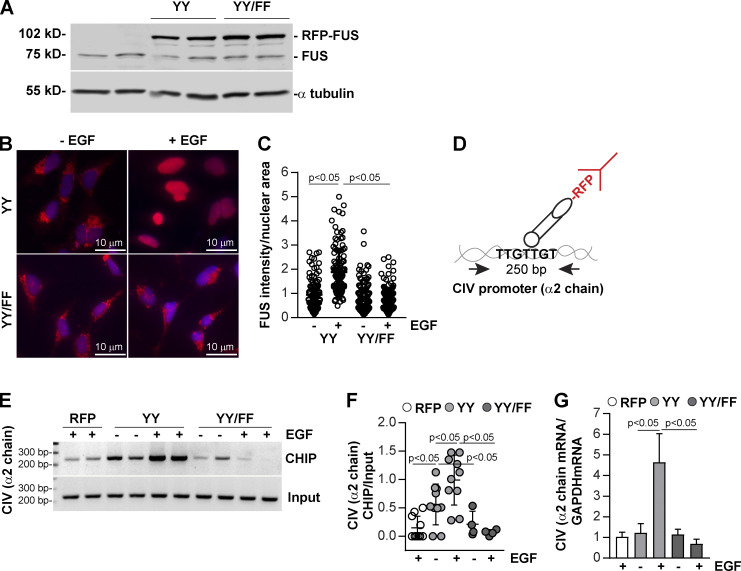
Figure 7.
FUS binds to the bidirectional promoter of collagen IV α1 and α2 chains and drives collagen IV α2 chain transcription. (A) HEK293 cells were transiently transfected with pRFP-C1 vector fused to WT FUS (YY) or FUS construct mutated in tyrosines 6 and 296 (YY/FF). After 48 h, cell lysates (20 µg/lane) were analyzed for the levels of endogenous and RFP-FUS (two transfections shown). (B) Serum-starved HEK expressing the RFP-FUS constructs described in A were left untreated or treated with EGF (20 ng/ml). After 1 h, the cells were fixed, permeabilized, and incubated with anti-RFP antibody followed by Alexa Fluor 555–conjugated secondary antibody. (C) Nuclear RFP intensity was analyzed using ImageJ, and values represent RFP intensity/nuclear area. Circles represent single nuclei (n = 115–124 with two experiments performed) and bars represent mean ± SD. (D) Overview of the ChIP assay performed in HEK cells expressing RFP empty vector (RFP), RFP-FUS (YY), or RFP-mutated FUS (YY/FF) constructs. (E) Cross-linked DNA–protein complexes from cells (n = 2 samples) untreated or treated with EGF (20 ng/ml) for 30 min were immunoprecipitated with RFP-TRAP beads. After DNA elution and purification, DNA fragments were amplified using collagen IV (CIV) α2 chain promoter primers spanning a putative FUS binding site. Input represents amplified CIV promoter in total DNA isolated from untreated or EGF-treated cells before immunoprecipitation. (F) CIV ChIP and input CIV bands were quantified by densitometry. Circles represent single values (n = 2–5 experiments performed in duplicate) and bars represent mean ± SD. (G) Serum-starved HEK expressing the constructs described in E were left untreated or treated with EGF (20 ng/ml). After 3 h, the levels of collagen IV α2 mRNA were analyzed by reverse transcription rqPCR. Bars and errors are mean ± SEM of three to six experiments performed in duplicate. One-way ANOVA and two-tailed t test were used for statistical analysis.