Abstract
Background
The lack of evidence-based recommendations for therapeutic decisions during the early weeks of the COVID-19 pandemic creates a unique scenario of clinical decision making which is worth to analyze. We aim to identify the drivers of therapeutic aggressiveness during the first weeks of the COVID-19 pandemic.
Methods
This cross-sectional worldwide survey (conducted April 12 to 19, 2020) was aimed at physicians who managed patients diagnosed with COVID-19. Treatment preferences were collected in five different clinical scenarios. We used multilevel mixed-effects ordered logistic regression to identify variables that were associated with the use of more aggressive therapies.
Findings
The survey was completed by 852 physicians from 44 different specialties and 29 countries. The heterogeneity of therapeutic decisions increased as the clinical scenario worsened. Factors associated with aggressive therapeutic decisions were higher self-perceived expertise (high vs. null, OR 1.95, 95%CI 1.31–2.89), perceived quality of COVID-19 publications (high vs. null, OR 1.92, 95%CI 1.17–3.16), and female sex (OR 1.17, 95%CI 1.02–1.33). Conversely, Infectious Diseases specialty, Latin American and North American origin, lower confidence in the treatments chosen, and having published articles indexed in PubMed as the first-author were associated with the use of less aggressive therapies.
Interpretation
Our study provides insight into the drivers of the decision-making process during a new and extreme health emergency. Different factors including the perceived expertise and quality of publications, gender, geographic origin, medical specialty and implication in medical research influenced this process. The clinical severity attenuated the physician's tolerance for uncertainty.
Funding
No funding was required.
Keywords: COVID-19, Clinical decision-making, Surveys and questionnaires, Therapeutics, Uncertainty
Research in context.
Evidence before this study
We searched Google Scholar without language restrictions with the terms “COVID19”, “SARS-CoV-2″, “evidence-based medicine” and “decision-making” for articles published until June 1, 2020. We selected studies in which the factors influencing the decision-making process in the absence of robust evidence were reported. We found mostly opinion articles and editorials dealing with the balance between decision-making during a new health crisis and evidence-based medicine, but no work that investigates the factors related to this decision-making process.
The lack of evidence-based recommendations for therapeutic decisions during the early weeks of the COVID-19 pandemic creates a unique scenario of clinical decision making which is worth to analyze.
Added value of this study
Our study provides a new insight into the drivers of the decision-making process during a new and extreme health emergency. Several factors including gender, geographic origin, self-perceived expertise, perceived quality of COVID-19 publications, clinical specialty, and involvement in medical research influence the decision-making process. The clinical severity affects the decision-making process, by attenuating the physician's tolerance for uncertainty.
Implications of all the available evidence
In the absence of evidence to guide decisions, a struggle between clinical intuition, emotions, rational thinking, and a constellation of low-quality information sources influence patient care. Awareness of the factors that affect our decision-making process during a new and extreme health emergency will help us to deliver better care to patients and to accelerate the set-up of clinical trials for the next pandemic.
Alt-text: Unlabelled box
1. Introduction
Current dogma in medicine indicates that clinical decisions should, as far as possible, be based on evidence [1,2]. To date, we lack solid evidence on the efficacy of any medication that has a positive outcome in COVID-19 patients. What were the drivers of therapeutic decisions during the first weeks of the health emergency caused by the SARS-CoV-2 outbreak? Decision-making during crisis management should be adaptive because sufficient evidence is often not available. In these cases, the compromise to implement different response measures may depend on evidence and other diverse factors such as narratives of colleagues, governmental messages, and the perceived severity of the disease risk, which, to some extent, is subjective and culturally determined [3,4]. While the healthcare systems were dramatically overwhelmed by the rampage of new COVID-19 cases, physicians throughout the world had to make therapeutic decisions in life-threatening situations with scarce and low-quality scientific evidence. As of August 2020, the vast majority of current treatment recommendations for COVID-19 are weak or very weak according to the GRADE framework [5], and as occurs when the certainty of the evidence is low, there is a close balance between the desirable and undesirable consequences or there is uncertainty in patient values and preferences [6]. Additionally, in many parts of the world, physicians from different specialties with very divergent areas of expertise have become part of multidisciplinary teams that have united in the fight against this infection, and in many cases, they are working outside their area of expertise [7].
Despite the absence of evidence-based recommendations for the use of any drug outside of a clinical trial, various medications such as hydroxychloroquine, azithromycin, lopinavir/ritonavir, monoclonal antibodies, and corticosteroids have been used off-label to try to minimize the impact of this infection. The demonstration of in vitro activity against coronaviruses and the potential effect that was observed in observational studies has led to the use of repurposed drugs and the approval of compassionate use drugs that have not been previously approved for clinical use [8]. During this unprecedented health emergency, therapeutic attitudes have been polarized on the rational–emotional scale. Some physicians have adopted an aggressive orientation, with a proactive attitude towards the use of several combinations of untested drugs or medication use based on weak evidence, which is consistent with the ‘do something’ principle [9], [10], [11], [12], [13]. Other healthcare professionals have assumed a conservative approach that is consistent with the Hippocratic Oath primum non nocere, clinical equipoise, and the evidence-based medicine dogma, maintaining a skeptical attitude when considering interventions that could cause harm [3,14,15].
We hypothesize that in a social and clinical emergency with scarce information to guide medical decisions, sociodemographic and professional factors and disease severity will influence the decision-making process. We aimed to identify the drivers of therapeutic aggressiveness during the first weeks of the COVID-19 pandemic.
2. Methods
2.1. Study design, participants, setting, and eligibility
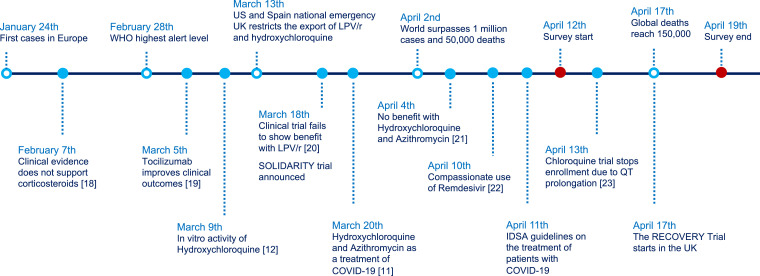
A survey in English was created in Google Forms format and disseminated worldwide to the medical community through social networks (mainly Twitter), professional networks, and personal contacts of the study investigators. We sent 55 emails to our professional network, focusing on international collaborators, and 14 tweets from 5 users, with an upper bound of 6500 followers and more than 30,000 cumulative interactions. The survey was aimed at physicians of any specialty who were involved in managing patients who were diagnosed with COVID-19. The survey allowed responses for 7 days, from April 12 to April 19, 2020, and collected socio-demographic and professional variables, including gender, year of birth, country of origin, country and city of residence, medical specialty, professional degree (trainee, specialist), academic degree (MD, PhD, assistant professor, full professor), level of perceived expertise in COVID-19, stratified number of patients with COVID-19 attended, previous diagnosis of COVID-19 in oneself or in a friend/relative, and type of workplace. In addition, we asked about the perceived quality of COVID-19 publications (null, low, medium, or high quality). Subsequently, physicians were faced with five different clinical scenarios, representing five examples of different clinical severity: (1) mildly symptomatic infection in an outpatient <65 years without comorbidity, without radiological involvement and with baseline oxygen saturation >95% on room air; (2) mildly symptomatic infection without pneumonia in a patient aged ≥65 years and/or comorbidity and/or oxygen saturation <95% on room air; (3) radiologically confirmed mild pneumonia, CURB-65 ≤ 1 [16] and oxygen saturation ≥95% on room air; (4) radiologically confirmed severe pneumonia who did not meet the acute respiratory distress syndrome (ARDS) criteria, CURB-65 >1 and/or oxygen saturation <95% on room air; (5) severe pneumonia who met the ARDS criteria, CURB-65 >1 and/or oxygen saturation <95% on room air. Treatment preferences for each scenario and the degree of confidence in the chosen therapeutic option were collected, using a five-point Likert scale [17]. Fig. 1 illustrates the main hallmarks of COVID-19 in the context of the survey timeframe. Because this study did not collect information from patients and this was an anonymous and voluntary survey that was addressed to physicians, the study did not require approval by the Ethics Committee, in accordance with the pertinent regulations.
Fig. 1.
Main hallmarks of COVID-19 in the context of the survey timeframe [18–[19], [20], [21], [22], [23]].
2.2. Statistical analysis
The descriptive analysis of participant characteristics and survey responses was performed using frequency distributions. We performed a correspondence analysis and used biplots for visual inspection of data matrices, projecting the different combinations of treatments that were chosen for each scenario in two-dimensional graphical form.
We arbitrarily defined ‘therapeutic aggressiveness’ according to the number of drugs that were used, with the exception of symptomatic treatment, which was not considered. The final variable was a seven-level ordinal variable (from zero to six drugs used). After inconclusive discussions with other specialists to define an aggressive treatment in the management of COVID-19 patients, and after exploring the combinations that were used in each clinical scenario, we thought that an ordinal scale based on the number of drugs used was, although arbitrary, the better solution to define therapeutic aggressiveness. To identify variables that were associated with the use of more aggressive therapies, we used multilevel mixed-effects ordered logistic regression with a random effect for each participant to allow for correlations that were caused by repeated responses by the same responder to the five clinical scenarios. Fixed effects included the sociodemographic, clinical, and professional-related variables that were collected in the survey, which, based on the investigators' knowledge of the field, could influence therapeutic decision-making. Missing data were less than 2% in all responses, and models used a complete-case analysis approach. Statistical analyses were performed using Stata v. 16.0 (StataCorp LP, College Station, TX, USA).
2.3. Role of funding source
This study represents authors' own work and no funding was required for its completion. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Characteristics of the respondents and decision making
The survey was completed by 852 physicians, and among them, 54% were women and the median age was 39 (interquartile range [IQR], 32–47) years. Most (86%) of the respondents had a residence in Spain, and 81% had a medical specialty, whereas 19% had a surgical or diagnostic specialty. Table 1 summarizes the participants’ characteristics. Supplementary Fig. S1 shows a map with the participants’ countries of residence. Their medical specialties are detailed in Supplementary Table S1.
Table 1.
Participants’ baseline characteristics (n = 852).
| Age, median (IQR), years | 39 (32– 47) |
|---|---|
| Gender, n (%) | |
| Male | 389 (46) |
| Female | 453 (54) |
| Non-binary | 2 (0.2) |
| Region of residence, n (%) | |
| Europe | 771 (93) |
| North America | 44 (5) |
| Latin America | 13 (2) |
| Other* | 5 (0.6) |
| Specialty, n (%) | |
| Medical | 681 (80) |
| Surgical | 136 (16) |
| Other† | 35 (4) |
| Professional degree, n (%) | |
| Trainee | 113 (16) |
| Specialist | 771 (84) |
| Academic degree, n (%) | |
| MD | 833 (100) |
| PhD | 273 (33) |
| Assistant professor | 114 (14) |
| Full professor | 24 (3) |
| Workplace, n (%) | |
| Large hospital (>800 beds) | 417 (49) |
| Medium hospital (400–800 beds) | 217 (26) |
| Small hospital (<400 beds) | 154 (18) |
| Primary Care (family practice) | 34 (4) |
| Private Clinic (private health insurance) | 22 (3) |
| Number of COVID-19 patients assessed, n (%) | |
| <10 | 119 (14) |
| 10–50 | 324 (38) |
| 51–200 | 313 (37) |
| >200 | 89 (11) |
Including African, Eastern Mediterranean, South-East Asia and Western-Pacific regions.
Other specialties include diagnostic and non-clinical specialties such as Radiology, Public Health, Pathology, Preventive Medicine, or Nuclear Medicine.
IQR, interquartile range.
The chosen treatments for each of the clinical scenarios, which were differentiated by the clinical severity, are summarized in Table 2. Symptomatic treatment without additional pharmacological intervention was the preferred therapy in scenario 1. However, in the other scenarios, chloroquine/hydroxychloroquine was the most chosen treatment followed by azithromycin. In scenario 5, 57% of the respondents chose pulse steroid therapy, and 72% of the respondents would prescribe a monoclonal antibody.
Table 2.
Chosen treatments by clinical scenario.
| Treatment | Clinical Scenario* |
||||
|---|---|---|---|---|---|
| 1 (n = 839) | 2 (n = 842) | 3 (n = 841) | 4 (n = 840) | 5 (n = 838) | |
| Symptomatic treatment only,% | 71 | 10 | 5 | 1 | 1 |
| Chloroquine/Hydroxychloroquine,% | 25 | 81 | 85 | 84 | 78 |
| Lopinavir/Ritonavir,% | 3 | 22 | 33 | 46 | 40 |
| Azithromycin,% | 14 | 50 | 60 | 62 | 57 |
| Remdesivir,% | 1 | 8 | 8 | 23 | 32 |
| Interferon beta-1b/alfa-2,% | 0 | 1 | 2 | 5 | 8 |
| Low-dose corticosteroids,% | 1 | 8 | 7 | 22 | 16 |
| Pulse steroid therapy (>250 mg of methylprednisolone or equivalent),% | 1 | 4 | 4 | 26 | 57 |
| Empirical antibiotic therapy,% | 2 | 1 | 29 | 48 | 54 |
| Tocilizumab or another monoclonal antibody,% | 1 | 4 | 4 | 33 | 72 |
| Mean number of drugs (SD) | 0.6 (1.2) | 2.1 (1.3) | 2.4 (1.2) | 3.6 (1.4) | 4.3 (1.4) |
SCENARIO 1: Mildly symptomatic infection in an outpatient <65 years without comorbidity, without radiological involvement and with baseline oxygen saturation >95% on room air.
SCENARIO 2: Mildly symptomatic infection without pneumonia in a patient aged ≥65 years and/or comorbidity and/or oxygen saturation <95% on room air.
SCENARIO 3: Radiologically confirmed mild pneumonia, CURB-65 score pneumonia severity index ≤1 and oxygen saturation ≥95% on room air.
SCENARIO 4: Radiologically confirmed severe pneumonia who did not meet the ARDS criteria, CURB-65 score pneumonia severity index >1 and/or oxygen saturation <95% on room air.
SCENARIO 5: Severe pneumonia who met the ARDS criteria, CURB-65 score pneumonia severity index >1 and/or oxygen saturation <95% on room air.
Abbreviations: ARDS, acute respiratory distress syndrome; SD, standard deviation.
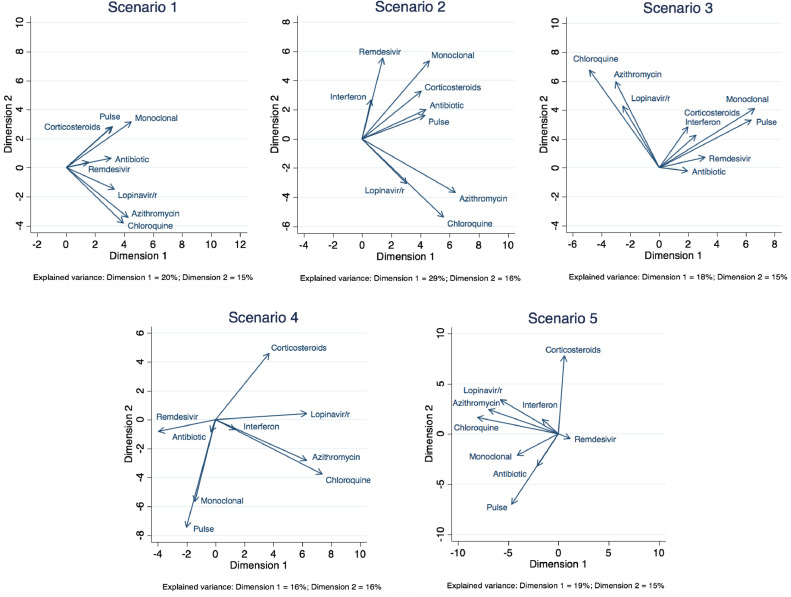
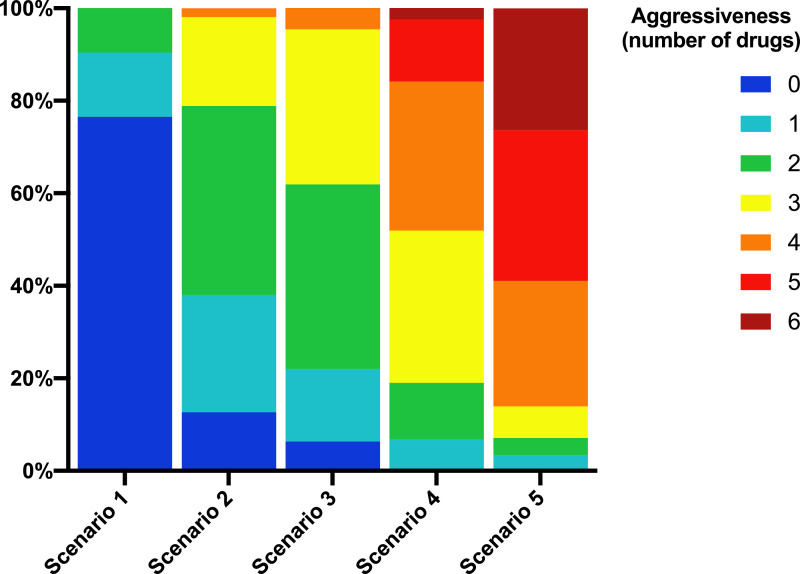
We found that there was a high variability in the responses, especially as the severity of the clinical scenario increased. Fig. 2 shows the two-dimensional correspondence plots representing the correlation between treatment options in each scenario. The treatment choices became more dissimilar as the clinical scenario worsened. There was a clear pattern of prescription favoring the combination of drugs either with supposed antiviral activity such as chloroquine, azithromycin, and lopinavir/ritonavir, or with presumed anti-inflammatory effects such as corticosteroids and monoclonal antibodies, which was maintained throughout the five simulated clinical scenarios (Fig. 2). Supplementary Fig. S2 represents the biplots that were extracted from the correspondence analysis. The number of chosen combinations tended to increase as the clinical scenario worsened. This number increased from 30 in scenario 1, to 79 in scenario 2, 88 in scenario 3, 160 in scenario 4, and 175 in scenario 5. In addition, the more the clinical scenario worsened, the greater the number of drugs that were used (Fig. 3). The treatment combinations that were chosen by at least ten participants for each scenario are listed in Supplementary Table S2.
Fig. 2.
Two-dimensional correspondence plots representing the correlation between treatment options in each scenario. The closer the arrows (smaller angles) in the orthogonal coordinates, the more correlated are the treatments. Uncorrelated treatments are represented by orthogonal vectors (angles close to 90°). Negatively correlated treatments are represented by vectors with angles close to 180°. The amount of variance explained by each component (or dimension) is presented as a footnote. In all scenarios, this total explained variance is small (lower than 50%).
Fig. 3.
Degree of aggressiveness category by scenario.
3.2. Use of non-evidence-based aggressive therapies
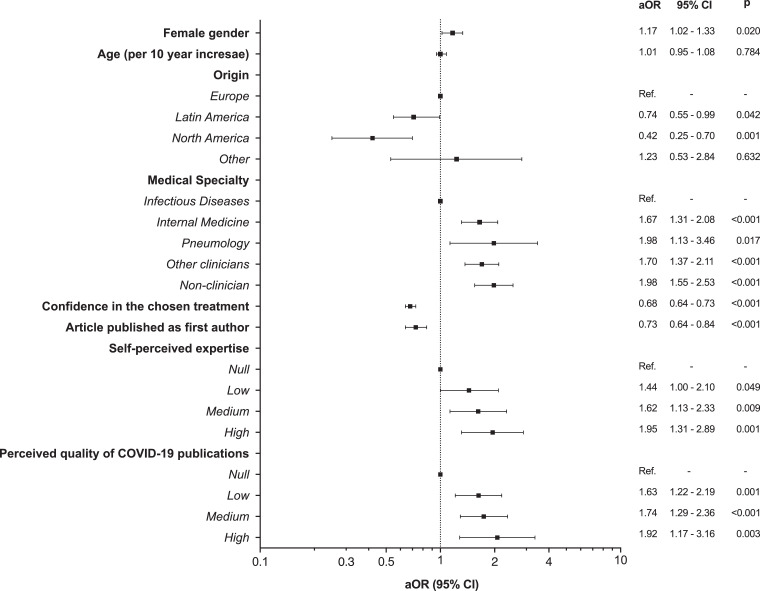
Using multivariable mixed-effects ordered logistic regression models, we evaluated the factors that were associated with the choice of more aggressive therapies. The factor with the greatest effect-size that was associated with a more aggressive therapeutic decisions was a higher self-confidence, which was captured as the self-perceived expertise (highly self-confident vs. null, odds ratio (OR) 1.95, 95% CI 1.31 to 2.89), followed by the perceived quality of COVID-19 publications, with a near two-fold increased probability of aggressive decisions for those who perceived the quality of COVID-19 publications to be high, compared to those who described the quality as null (OR 1.92, 95% CI 1.17 to 3.16). Compared to infectologists, other clinical specialists that also had a high healthcare burden in this epidemic, such as internists and pneumologists, made more aggressive decisions (Internal Medicine, OR 1.67, 95% CI 1.31 to 2.08; Pneumology, OR 1.98, 95% CI 1.13 to 3.46). We also found a weak effect of sex because women were more likely to make aggressive decisions (OR 1.17, 95% CI 1.02 to 1.33). Conversely, physicians of Latin American and North American origin showed a lower confidence in the treatments that were chosen, and having published articles indexed in PubMed as a first-author was associated with the use of less aggressive therapies (Fig. 4).
Fig. 4.
Variables associated with the use of more aggressive therapies in the multilevel mixed-effects ordered logistic regression. aOR, adjusted odds ratio; CI, confidence interval; Ref, reference category.
4. Discussion
This research reflects the choice of therapy during an unprecedented pandemic, in which evidence about the effectiveness of therapeutic strategies has been scarce. We found that several factors including self-perceived expertise, quality of COVID-19 publications as determined by the physician, clinical specialty, gender, geographic origin, and involvement in medical research influenced the decision-making process. We found that the heterogeneity of therapeutic decisions increased as the clinical scenario worsened, indicating that the clinical severity attenuated the physician's tolerance for uncertainty and affected the decision-making process.
Management for SARS-CoV-2 has widely been supportive, and during the first weeks of the pandemic, no specific therapy had been scientifically proven to reduce mortality (Fig. 1). In many countries, physicians who were caring for patients with COVID-19 have faced an unprecedented health emergency with overwhelmed healthcare systems that were confronted with a new and often life-threatening disease. Most physicians who were involved in the management of patients with COVID-19 have witnessed passionate debates that were polarized between a clear disposition to try new therapeutic options based on anecdotical reports or personal experiences and a more cautious approach to at least avoid harm in the absence of efficacy data. The factors that we identified as predictors of a more conservative approach suggest an association with a greater sensitivity to a rational and evidence-based decision-making process (i.e. more criticism of the published literature, more involvement as leading authors on PubMed-indexed articles). We also found an effect of the region of residence, especially for physicians working in Europe compared with the US, with the former being more conservative in their decisions. The European countries that contributed most to the survey (Spain, Italy, and the United Kingdom) were those that were more strongly affected by SARS-CoV-2 when this survey was conducted. We think that these geographic differences indicate a more paternalist pattern of decision-making in Europe compared to the US and possibly a greater constraint to prescribe off-label medications in the US that is motivated by a greater fear of medical malpractice litigation [24]. Additionally, our data suggest different clinical management among infectious disease physicians compared to other specialists. To date, we have no data demonstrating the association between infectious disease physician care and the clinical outcomes of COVID-19 [25]. This more conservative approach among infectious disease physicians could be explained by the greater acceptance of uncertainty with a new infectious disease, but also by awareness of the potential deleterious consequences of off-label medications that were used in past infectious diseases, as observed with ribavirin in the last SARS-CoV outbreak in 2003 [26].
Our study has some limitations. The first limitation is selection bias. We disseminated the survey through personal and professional accounts on social platforms, mainly Twitter, and through personal contacts by email, so the study population is essentially representative of physicians using these tools and involved in the management of patients during the first two months of the SARS-CoV-2 pandemic in western Europe. In addition, the low prevalence of participants out of Europe warrants a cautious interpretation of the differences found between geographical areas. Second, because of the methods that were used for survey dissemination, we cannot estimate the rate of participation. Third, the fact that the clinical decisions analyzed were determined by confronting clinicians with different clinical scenarios in a survey must be taken in consideration when interpreting our results, since a number of factors, including recall bias or exposure to new information may have resulted in discrepancies with respect to the real decisions made. Last, in the absence of a ‘therapeutic aggressiveness’ definition that was specific for COVID-19, we arbitrarily created a scale based on the number of drugs that were administered. Admittedly, regardless the disease severity, an inappropriate drug could be considered to be an inadequate decision.
We planned this study because we were intrigued by the therapeutic decision-making patterns that were perceived early in the COVID-19 pandemic. We were concerned by the anecdotical reports, ‘expert’ advice, and clinical discussions on social platforms such as Twitter that seemed to influence clinical reasoning [15]. We were also intrigued by how faith in unproven treatments could undermine their implementation and recruitment for clinical trials. The urgent need of clinical guidance in a moment with very scarce peer-reviewed information, and a wave of non-peer reviewed papers, press-releases, and controversial results in small cohort studies assessing surrogate end-points of efficacy [11,26] have nurtured clinical practice guidelines that sometimes have low reliability, and thus, make clinical practice very heterogeneous. In addition, during a health emergency, clinical trials may be thought to be unfeasible or even unethical, and, for many, the need for some evidence often outweighs the rigor of science. For example, a call for attention has been made about the flawed methodology and suboptimal reporting of research on chloroquine/hydroxychloroquine for the treatment of COVID-19 patients during the early phases of the pandemic [27].
In the absence of evidence to guide decisions, a struggle between clinical intuition, emotions, rational thinking, and a constellation of low-quality information sources influenced patient care. Our study fuels the debate about how we could have provided better guidance to physicians during the COVID-19 pandemic. Awareness of the factors that affect our decision-making process during a new and extreme health emergency will help us to deliver better care to patients and to accelerate the set-up of clinical trials for the next pandemic.
Authors’ contributions
J.M-S. and S.S-V., conceptualized the study; J.A.P-M. and S.M. performed the literature search, J.Z. planned the statistical strategy, J.M-S., J.Z. and S.S.-V. analyzed the data and generated the figures, and wrote the first draft of the manuscript, J.A.P-M. and S.M contributed to writing and provided a critical review of the manuscript. All authors revised and approved the final manuscript.
Declaration of Competing Interest
There are no potential conflicts of interest.
Acknowledgments
Acknowledgements
We thank all healthcare workers for their exemplary commitment against COVID-19.
Funding
This study represents authors' own work and no funding was required for its completion.
Data sharing statement
All data requests should be submitted to the corresponding author for consideration. Data will be accessible upon reasonable request to achieve aims in the approved proposal. Access to anonymised data may be granted following review.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100539.
Appendix. Supplementary materials
References
- 1.Evidence-based medicine: a new approach to teaching the practice of medicine. JAMA. 1992;268:2420–2425. doi: 10.1001/jama.1992.03490170092032. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg W., Donald A. Evidence based medicine: an approach to clinical problem-solving. BMJ. 1995;310:1122. doi: 10.1136/bmj.310.6987.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Centre for Disease Prevention and Control . ECDC; Stockholm: 2019. The use of evidence in decision-making during public health emergencies. [DOI] [Google Scholar]
- 4.Baden L.R., Rubin E.J., Morrissey S. We can do better - Improving outcomes in the midst of an emergency. N Engl J Med. 2017;377:1482–1484. doi: 10.1056/NEJMe1712330. [DOI] [PubMed] [Google Scholar]
- 5.Guyatt G.H., Oxman A.D., Vist G.E. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso-Coello P., Oxman A.D., Moberg J. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: clinical practice guidelines. BMJ. 2016;353:i2089. doi: 10.1136/bmj.i2089. [DOI] [PubMed] [Google Scholar]
- 7.Borsa S., Bertani G., Pluderi M. Our darkest hours (being neurosurgeons during the COVID-19 war) Acta Neurochir (Wien) 2020;162:1227–1228. doi: 10.1007/s00701-020-04333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalil A.C. Treating COVID-19 - Off-label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA. 2020;323:1897–1898. doi: 10.1001/jama.2020.4742. [DOI] [PubMed] [Google Scholar]
- 9.Rome B.N., Avorn J. Drug evaluation during the Covid-19 pandemic. N Engl J Med. 2020;382:2282–2284. doi: 10.1056/nejmp2009457. [DOI] [PubMed] [Google Scholar]
- 10.Kim A.H.J., Sparks J.A., Lie J.W. A rush to judgment? Rapid reporting and dissemination of results and Its consequences regarding the use of hydroxychloroquine for COVID-19. Ann Intern Med. 2020;172:819–821. doi: 10.7326/M20-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gautret P., Lagier J.-.C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [Ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao X., Ye F., Zhang M. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;2:1–25. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin E.J., Harrington D.P., Hogan J.W. The urgency of care during the Covid-19 pandemic — Learning as we go. N Engl J Med. 2020;382:2461–2462. doi: 10.1056/nejme2015903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freedman B. Equipoise and the ethics of clinical research. N Engl J Med. 1987;317:141–145. doi: 10.1056/NEJM198707163170304. [DOI] [PubMed] [Google Scholar]
- 15.Rice T.W., Janz D.R. In defense of evidence-based medicine for the treatment of COVID-19 ARDS. Ann Am Thorac Soc. 2020 doi: 10.1513/AnnalsATS.202004-325IP. [Ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim W.S., Van Der Eerden M.M., Laing R. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chyung S.Y.Y., Roberts K., Swanson I. Evidence-based survey design: the use of a midpoint on the Likert scale. Perf Improv. 2017;56:15–23. doi: 10.1002/pfi.21727. [DOI] [Google Scholar]
- 18.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X., Han M., Li T. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao B., Wang Y., Wen D. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molina J.M., Delaugerre C., Le Goff J. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 2020;50:384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grein J., Ohmagari N., Shin D. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg E.S., Dufort E.M., Udo T. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA. 2020;323:2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill P. Off licence and off label prescribing in children: litigation fears for physicians. Arch Dis Child. 2005;90:i17–i18. doi: 10.1136/adc.2004.058867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walensky R.P., McQuillen D.P., Shahbazi S. Where is the ID in COVID-19? Ann Intern Med. 2020:M20–2684. doi: 10.7326/M20-2684. [Ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye X.T., Luo Y.L., Xia S.C. Clinical efficacy of lopinavir/ritonavir in the treatment of Coronavirus disease 2019. Eur Rev Med Pharmacol Sci. 2020;24:3390–3396. doi: 10.26355/eurrev_202003_20706. [DOI] [PubMed] [Google Scholar]
- 27.Alexander P.E., Debono V.B., Mammen M.J. COVID-19 coronavirus research has overall low methodological quality thus far: case in point for chloroquine/hydroxychloroquine. J Clin Epidemiol. 2020;123:120–126. doi: 10.1016/j.jclinepi.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.