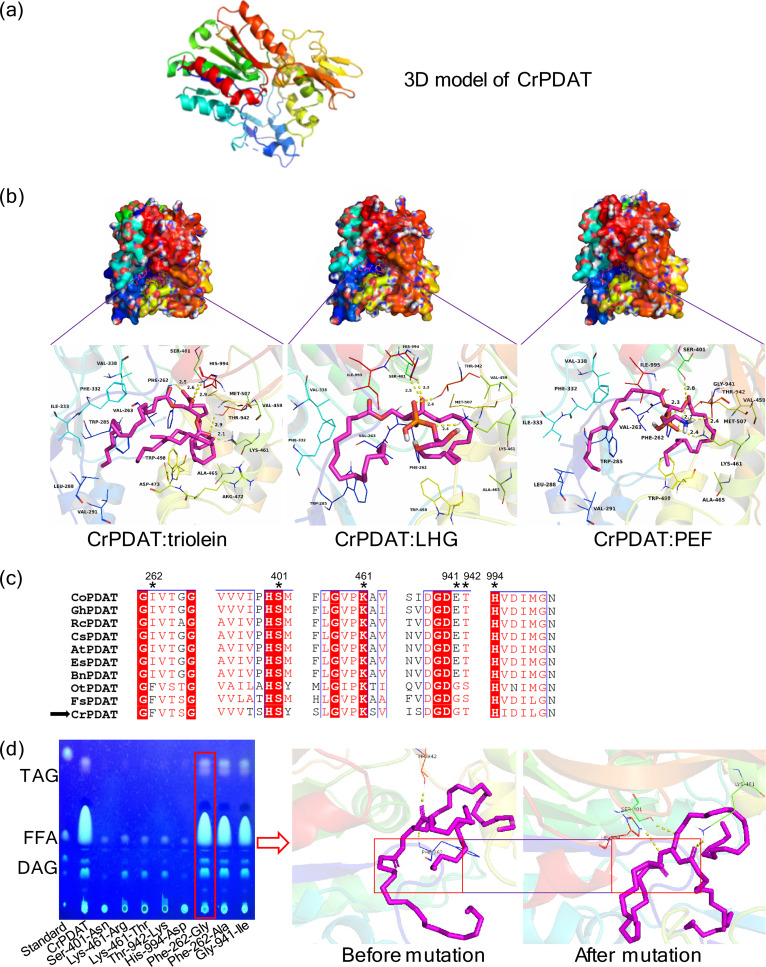
FIG 5.
Schematic of the rational mutagenesis system workflow. The aim of this system is to decrease TAG lipase activity without impairing acyltransferase activity of CrPDAT (a). Three docking models of triolein (left), LHG (middle), and PEF (right) to CrPDAT were simulated (b). The essential amino acids involved in hydrogen bond formation were predicted. Sequence alignment of 10 selected PDATs was carried out to identify the distribution of those predicted essential amino acids (c). Identical residues are shown in white on a red background, while similar residues are shown in red. The predicted binding sites are indicated by black asterisks. The ability of the rational designed mutants to accumulate TAG was determined by TLC (d). The binding model of the positive mutant (Phe-262-Gly) was reconstructed by molecular docking to confirm the loss of the hydrogen bond between CrPDAT and triolein (d).