ABSTRACT Acinetobacter baumannii
(A. baumannii) is a pathogen responsible for nosocomial infections among the hospitalized patients. The aim of this study was to investigate genotyping and molecular characterization and to examine the biofilm formation ability of A. baumannii isolates. In total, 70 A. baumannii isolates were collected from patients admitted to Imam Khomeini Hospital in Ahvaz, Southwestern Iran. Minimum inhibitory concentrations (MIC) test was performed using Vitek 2 system. The presence of genes encoding metallo-β-lactamases, oxacillinases, and integrase and the biofilm formation ability were then evaluated. Multiple locus variable-number tandem repeat (VNTR) analysis (MLVA) typing and multiplex PCR were performed to determine the genetic relationships. The blaOXA-23-like gene had the highest prevalence. The frequency of genes encoding blaSPM, blaIMP, and blaVIM among MDR A. baumannii isolates were 12 (17.1%), 18 (25.7%), and 22 (31.4%), respectively. Moreover, 46 isolates (75.4%) harbored class I integron and 10 isolates (16.39%) carried class II integron. The number of weak, moderate and strong biofilm-producing isolates were 3 (4.3%), 7 (10%), and 55 (78.5%), respectively. The results showed that 70 A. baumannii isolates were grouped into 12 distinct MLVA types with five clusters and four singleton genotypes. In addition, 25 (35.7%) isolates were assigned to international clone (IC) variants, 37 (52.8%) isolates belonged to group 1 (IC II), and 8 (11.4%) isolates belonged to group 2 (IC I). Our findings revealed that the population structure of the A. baumannii isolates was genetically diverse. More focus on genetic variation and antibiotic resistance of A. baumannii isolates are recommended.
KEYWORDS: Acinetobacter baumannii, drug resistance, integron, metallo-β-lactamase, MLVA
Introduction
Acinetobacter baumannii is a ubiquitous bacterium known to be implicated in nosocomial infections, particularly in hospitalized patients. Infection caused by this hospital-associated pathogen often leads to significant morbidity and mortality [1]. Clinical isolates of A. baumannii have the ability to remain in hospital environments, persistently, for a prolonged period of time, commonly giving rise to hospital outbreaks [2].
In previous decades, A. baumannii resistance to various antimicrobial drugs has markedly escalated. This pattern of antibiotic resistance frequently varies in time and from one area to another or even within the same area [3,4]. Recently, multidrug-resistant (MDR) A. baumannii strains have emerged as formidable nosocomial pathogens. In many developing countries, doctors face serious problems in the management of patients infected with MDR A. baumannii, imposing considerable challenges on healthcare systems owing to treatment failure and increased mortality [5,6]. Carbapenem antibiotics are the most appropriate drugs of choice for treating infections caused by MDR A. baumannii strains. Overuse of broad-spectrum carbapenems and/or cephalosporins is a major risk factor for colonization or infection with carbapenemase-producing A. baumannii strains. Unfortunately, the prevalence of carbapenem resistance in the aforementioned strains is increasingly growing [7].
The most significant mechanisms of resistance to antibiotics include target protection or modification, antibiotic enzymatic degradation, decreased permeability, and drug extrusion by the efflux pump. Resistance to carbapenems in A. baumannii is related to a variety of enzymes, including metallo-β-lactamases (IMP, VIM, and NDM) and OXA-type carbapenemases (OXA-24, OXA-58, OXA-51, and OXA-23) [8,9]. MDR A. baumannii infections are typically associated with high mortality, and patients acquire infection from other patients and from different environmental sources [10]. Some clinical isolates of A. baumannii have a high propensity for biofilm formation and occasionally cause medical device-related infections. Biofilm formation is an important pathogenic mechanism in such infections and has a key role in the long-term survival of bacteria under environmental pressures [11].
Based on epidemiological studies and population genetic investigations of A. baumannii, there are several different typing methods, including multiple-locus variable number tandem repeats (VNTRs) analysis (MLVA), pulsed-field gel electrophoresis (PFGE), whole genome sequencing (WGS), multilocus sequence typing (MLST), and PCR-based sequence group (SG) profiling [12–14]. This study aimed to investigate the presence of genes encoding metallo-β-lactamases, oxacillinases, integrase and to scrutinize the biofilm formation ability of A. baumannii isolates. MLVA typing and multiplex PCR were used to determine the genetic relationships of the isolates collected from patients admitted to Imam Khomeini Hospital in Ahvaz, Southwestern Iran.
Materials and methods
Bacterial isolates and identification
This cross-sectional study was confirmed by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (no. IR.AJUMS.REC.1397.793) in accordance with the Declaration of Helsinki and conducted between October 2018 and July 2019. Before study initiation, written informed consents were provided by all the patients. A total of 70 non-repetitive A. baumannii isolates were collected from clinical samples (tracheal aspirates, blood, cerebrospinal fluid [CSF] urine, catheter, and pleural fluid) of patients who were referred to Imam Khomeini Hospital in Ahvaz. The isolates were initially characterized by standard tests, and their final identification was performed by PCR of blaOXA-51-like gene and multiplex of gyrB. The A. baumannii ATCC19606 was selected as a positive control [15,16].
Antimicrobial susceptibility testing
The minimum inhibitory concentrations (MICs) of 17 antibiotics (ampicillin-sulbactam, piperacillin, cefepime, ceftazidime, cefotaxime, amikacin, meropenem, imipenem, ciprofloxacin, levofloxacin, minocycline, gentamicin, tobramycin, tetracycline, colistin, tigecycline, and trimethoprim-sulfamethoxazole) against A. baumannii isolates were determined using Vitek 2 system (bioMérieux, Marcy l’Etoile, France). The MICs of all the antibiotics were interpreted by the mentioned system according to the CLSI guidelines [17]. The MIC50 and MIC90 of each antibiotic were then calculated.
The MIC breakpoints of resistance were ≥ 4 µg/ml for colistin and ≥ 8 µg/ml for imipenem and meropenem. For tigecycline, no MIC interpretive breakpoint was recommended by the EUCAST and CLSI. MIC ≥ 8 μg/ml was proposed as the resistant breakpoint according to the criteria suggested by Jones et al. [18]. MDR Acinetobacter isolates are defined as strains resistant to at least three classes of antimicrobial agents, including all penicillins and cephalosporins, aminoglycosides, and fluoroquinolones [19]. Quality control for the MIC analysis was performed with Escherichia coli ATCC 25,922 and Pseudomonas aeruginosa ATCC 27,853, and the phenotypic detection of MBL production was accomplished as per a former study [20].
PCR amplification of the β-lactamases-encoding genes and identification of integrons
Detection of genes encoding oxacillinases (blaOXA-23-like, blaOXA-24-like, blaOXA-51-like, blaOXA-58-like, and blaOXA-143-like) [21] and bla genes (blaSIM, blaNDM, blaVIM, blaIMP, and blaSPM) was conducted by using the specific primers described previously [22,23]. Bacterial DNA was obtained by the boiling method [23].
PCR was performed for each pair of primers in a total volume of 25 µl in a reaction tube containing 2 µl of DNA template, 10 µl of Amplicon Master Mix, 1 µl of each forward and reverse primer (2.5 pmol), and 11 µl of distilled water. PCR reactions included 30 cycles of amplification in a Mastercycler (Eppendorf, Germany) under the following conditions: denaturation at 95 °C for five min, annealing at 55 °C for 30 s, and extension at 72 °C for 45 s, with a final extension at 72 °C for 6 min. Amplified products were visualized following electrophoresis on a 1% agarose gel stained with safe stain in a Tris-Borate-EDTA (TBE) buffer (Promega, USA). PCR was also performed to detect the presence of class I, II, and III integrons by the amplification of integrase genes, including intI1-, intI2-, and intI3-specific primers [24].
In vitro bacterial biofilm assay
The biofilm formation ability of the A. baumannii isolates on 96-well polystyrene microtiter plates (MTP) was assessed using the crystal violet staining (CVS) method, as formerly described [25]. For evaluating biofilm formation, Muller Hinton Broth and A. baumannii ATCC19606 were used as negative and positive controls, respectively. The results were interpreted according to the criteria described by Zhang et al. [26]. The isolates were strong biofilm producers when 4 × ODc < OD570, moderate biofilm producers when 2 × ODc < OD570 ≤ 4 × ODc, weak biofilm producers when ODc < OD570 ≤ 2 × ODc, and no biofilm producers when OD570 ≤ ODc. All the experiments were conducted in triplicate.
Molecular typing methods
For molecular typing, two multiplex PCRs were performed to selectively amplify the alleles of chaperone-subunit usher E (csuE), the outer membrane protein A (ompA), and the intrinsic carbapenemase (blaOXA-51-like) gene, and to determine the sequence groups and the corresponding major international clones (ICs) I–III according to Turton et al. [27]. The amplification reaction was carried out by using a thermal cycler (Mastercycler Eppendorf, Germany) with an initial denaturation at 94 °C for 3 min, followed by 30 cycles of 94 °C for 45 s, 57 °C for 45 s, and 72 °C for 1 min, and a final extension step was conducted at 72 °C for 5 min. The MLVA typing of A. baumannii isolates was carried out using the MLVA-8 scheme method designed by Pourcel et al. [13]. The MLVA-8 scheme profiles in each isolate were identified by the number of repeats estimated at each VNTR locus.
Statistical analysis
Descriptive data were analyzed using Microsoft excel and SPSS version 22 statistics software (IBM Corporation, Armonk, NY, USA). Fisher’s exact test was applied to analyze intergroup significance. In addition, p < 0.05 was considered as statistical significance level. The results are presented as descriptive statistics in terms of relative frequency.
Results
Studied population, sampling, and antibiotic susceptibility
In the current study, 70 non-duplicative A. baumannii isolates were collected from 42 (60%) males and 28 (40%) females with the mean age of 42.5 ± 10 (ranged 5–80) years, with the maximum number of cases in the age group of 41 to 60 years (n = 36). These isolates were obtained from different clinical specimens, including tracheal aspirates (26; 37.1%), blood (9; 12.8%), CSF (5; 7.1%), urine (8; 11.4%), catheter (15; 21.4%), and pleural fluid (7; 10%). The frequencies of the A. baumannii isolates collected from different wards were 31 (44.2%) in the intensive care unit (ICU), as well as 10 (14.3%) in surgery, 8 (11.5%) in urology, 6 (8.6%) in infectious diseases, 7 (10%) in general, 5 (7.1%) in pediatric, and 3 (4.3%) in neurology units (Table 1).
Table 1.
Clinical data of the patients with A. baumannii isolates. ID: infectious diseases; ICU: intensive care unit; M: male; F: female; TA: tracheal aspirates; CSF: Cerebrospinal fluid; PF: pleural fluid.
| Strain ID | Ward | Gender | Clinical samples | Date of collection |
|---|---|---|---|---|
| Ab 58 | General | F | TA | May 2018 |
| Ab 59 | Surgery | M | PF | May 2018 |
| Ab 66 | ICU | F | CSF | June 2019 |
| Ab 43 | Neurology | F | Catheter | February 2019 |
| Ab 45 | Urology | M | Blood | February 2019 |
| Ab 46 | General | M | TA | March 2019 |
| Ab 48 | Surgery | F | TA | March 2019 |
| Ab 49 | ID | F | TA | March 2019 |
| Ab 50 | ICU | M | Catheter | March 2019 |
| Ab 51 | Pediatric | M | TA | March 2019 |
| Ab 52 | Urology | F | Urine | April 2019 |
| Ab 53 | ICU | M | Blood | April 2019 |
| Ab 54 | ICU | M | Catheter | April 2019 |
| Ab 60 | ID | M | Catheter | May 2018 |
| Ab 61 | ICU | F | TA | May 2018 |
| Ab62 | ICU | F | TA | May 2018 |
| Ab 63 | ICU | M | Blood | June 2019 |
| Ab 64 | General | M | Catheter | June 2019 |
| Ab 67 | ID | M | Urine | June 2019 |
| Ab 68 | ICU | M | PF | June 2019 |
| Ab 69 | ICU | F | CSF | June 2019 |
| Ab 70 | Urology | F | Catheter | June 2019 |
| Ab 65 | Surgery | M | Catheter | June 2019 |
| Ab 15 | ICU | F | Catheter | November 2018 |
| Ab 16 | Pediatric | M | Blood | December 2018 |
| Ab 17 | ICU | F | TA | December 2018 |
| Ab 18 | ICU | M | Catheter | December 2018 |
| Ab 19 | Urology | F | TA | December 2018 |
| Ab 20 | ID | F | PF | December 2018 |
| Ab 21 | ICU | M | Blood | December 2018 |
| Ab 22 | Surgery | M | TA | December 2018 |
| Ab 23 | Neurology | F | Catheter | December 2018 |
| Ab 24 | General | M | TA | December 2018 |
| Ab 25 | ICU | M | Catheter | December 2018 |
| Ab 26 | ID | F | Blood | December 2018 |
| Ab 27 | ICU | M | TA | December 2018 |
| Ab 28 | Surgery | M | Blood | December 2018 |
| Ab 1 | ICU | M | Urine | October 2018 |
| Ab 2 | ICU | F | PF | October 2018 |
| Ab 3 | Urology | M | Blood | October 2018 |
| Ab 4 | ICU | F | Catheter | October 2018 |
| Ab 7 | General | M | Blood | October 2018 |
| Ab 5 | Pediatric | F | TA | October 2018 |
| Ab 8 | ICU | M | TA | October 2018 |
| Ab 6 | ICU | M | CSF | October 2018 |
| Ab 10 | Surgery | M | PF | October 2018 |
| Ab 11 | Neurology | M | CSF | November 2018 |
| Ab 12 | ICU | F | Catheter | November 2018 |
| Ab 13 | Surgery | M | Urine | November 2018 |
| Ab 14 | General | M | TA | November 2018 |
| Ab 9 | Urology | F | Urine | October 2018 |
| Ab 44 | ICU | M | TA | February 2019 |
| Ab 47 | ICU | M | Urine | March 2019 |
| Ab 55 | Urology | M | TA | April 2019 |
| Ab 29 | ID | F | Catheter | January 2019 |
| Ab 30 | Pediatric | M | TA | January 2019 |
| Ab 31 | ICU | M | PF | January 2019 |
| Ab 32 | Urology | F | TA | January 2019 |
| Ab 33 | ICU | M | TA | January 2019 |
| Ab 34 | Surgery | F | Urine | January 2019 |
| Ab 35 | ICU | F | Catheter | January 2019 |
| Ab 36 | General | M | TA | January 2019 |
| Ab 37 | Pediatric | M | TA | January 2019 |
| Ab 38 | Surgery | M | Urine | February 2019 |
| Ab 39 | ICU | M | CSF | February 2019 |
| Ab 40 | ICU | F | TA | February 2019 |
| Ab 41 | Surgery | F | TA | February 2019 |
| Ab 42 | ICU | M | PF | February 2019 |
| Ab 56 | ICU | F | TA | May 2018 |
| Ab 57 | ICU | M | TA | May 2018 |
According to antibiotic susceptibility testing, among 70 A. baumannii isolates screened, 61 (87.1%) were MDR and 9 (12.8%) were extensive drug-resistant (XDR) isolates. The results showed 100% resistance to ceftazidime, cefepime, and cefotaxime, indicating no effect on A. baumannii isolates, while colistin and tigecycline were the most active antimicrobial agents (with 2.8% and 45.7% resistance, respectively). Besides, among A. baumannii isolates, resistance to other antibiotics was ≥ 50% (Table 2).
Table 2.
Antibiotic susceptibility pattern of A. baumannii isolates.
| Antibiotic | GEN | TOB | LEV | TET | MIN | CTZ | CFP | PIP | TGC | AMP/S | COL | CTX | SXT | AMK | CIP | IMI | MER | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Susceptibility pattern | RN (%) | 58 (82.8) | 52 (74.3) | 65 (92.8) | 54 (77.1) | 43 (61.4) | 70 (100) | 70 (100) | 68 (97.1) | 32 (45.7) | 55 (78.6) | 2 (2.8) | 70 (100) | 67 (95.7) | 59 (84.2) | 65 (92.8) | 50 (71.4) | 52 (74.2) |
| IN (%) | 2 (2.8) | 4 (5.7) | 0 (0.0) | 0 (0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 2 (2.8) | 0(0.0) | 3 (4.3) | 0 (0.0) | |
| SN (%) | 10 (14.3) | 14 (20) | 5 (7.1) | 16 (22.8) | 27 (38.5) | 0(0.0) | 0(0.0) | 2(2.8) | 38 (54.3) | 15(21.4) | 68 (97.1) | 0(0.0) | 3 (4.3) | 9 (12.8) | 5 (7.1) | 17 (24.2) | 18 (25.7) | |
| Interpretive categories and MIC breakpoints (µg/ml) | R | ≥16 | ≥16 | ≥8 | ≥16 | ≥16 | ≥32 | ≥32 | ≥128 | ≥8 | ≥32/16 | ≥4 | ≥64 | ≥4/76 | 64 | ≥4 | ≥8 | ≥8 |
| I | 8 | 8 | 4 | 8 | 8 | 16 | 16 | 32–64 | - | 16/8 | - | 16–32 | - | 32 | 2 | 4 | 4 | |
| S | ≤4 | ≤4 | ≤2 | ≤4 | ≤4 | ≤8 | ≤8 | ≤16 | <8 | ≤8/4 | ≤2 | ≤8 | ≤ 2/38 | 16 | ≤1 | ≤2 | ≤2 | |
| Range | 2 to ≥240 | 1 to ≥240 | 1 to ≥ 240 | 2 to 256 | 2 to 256 | 4 to ≥ 256 | 4 to ≥ 256 | 5 to ≥ 240 | 0.25 to 64 | 2/1 to ≥ 256/128 | 0.125 to 32 | 4 to ≥ 256 | 5 to 240 | 8 to ≥ 256 | 0.1 to ≥ 240 | 0.25 to 256 | 0.25 to 256 | |
GEN: gentamicin; TOB: tobramycin; LEV: levofloxacin; TET: tetracycline; MIN: minocycline; CTZ: ceftazidime; CFP: cefepime; PIP: piperacillin; TGC: tigecycline; Amp/S: ampicillin/Sulbactam; COL: colistin; CTX: cefotaxime; SXT: trimethoprim-sulphamethoxazole; AMK: amikacin; CIP: ciprofloxacin; IMI: imipenem; MEM: meropenem; R: resistant; I: intermaediate; S: susceptible.
All XDR isolates had blaOXA-23-like gene, and all belonged to clone 1, 2 or 10. Two A. baumannii isolates were resistant to colistin (MIC = 32 and 8 µg/mL), had strong biofilm formation, were XDR phenotype and belonged to ICs 1 and 2. 41 (58.5%) isolates were simultaneously resistant to imipenem and meropenem, and 52 (74.2%) out of 70 A. baumannii isolates were carbapenem-resistant (CRAB). All meropenem- and imipenem-resistant isolates contained at least one blaOXA-24-like and/or blaOXA-23-like carbapenemase. Among the meropenem-sensitive and -resistant isolates, 18 (25.7%) and 52 (74.2%) isolates had MICs ranging from 0.25 to ≤ 2 and from ≥ 8 to 256 µg/ml, respectively. The MICs for imipenem ranged from 0.25 to ≤ 2 µg/mL in 17 (24.2) isolates, while 50 (71.4%) isolates had MICs ≥ 8–256 µg/ml for this antibiotic. Three isolates also showed MICs in the intermediate range (4 µg/ml) for imipenem (Table 2).
Frequency of β-lactamases-encoding genes and characterization of integrons
PCR results displayed the presence of blaOXA-51-like gene in all the isolates, whereas no isolate harbored blaOXA-143-like gene. The blaOXA-23-like gene had the highest frequency (46 isolates, 65.7%). The combination of blaOXA-23-like/blaOXA-24-like and blaOXA-23-like/blaOXA-24-like/blaOXA-58-like genes were found in 8 (11.4%) and 3 (4.2%) isolates, respectively. The coexistence of the blaOXA-23-like and blaOXA-58-like genes occurred only in 6 isolates (8.5%).
The frequency rates of the genes encoding blaSPM, blaIMP, and blaVIM among A. baumannii isolates were 12 (17.1%), 18 (25.7%), and 22 (31.4%), respectively. The blaVIM gene coexisted with blaIMP in 9 (12.8%) isolates; however, blaNDM and blaSIM were not identified in these isolates. Among CRAB isolates, 41 were phenotypically recognized as MBL-producing isolates, of which 8, 16, and 11 strains were positive for the blaSPM, blaVIM, and blaIMP genes, and no other MBLs were detected. Also, in the remaining 6 (12.8%) isolates, the blaVIM gene coexisted with blaIMP.
PCR amplification of integron genes showed that 46 isolates (75.4%) harbored class I integron, and 10 isolates (16.39%) carried class II integron. Class III integron was not identified in any isolates. Meanwhile, 5 isolates (8.19%) had both classes I and II integrons, and 9 isolates did not have these integron genes. Antibiotic susceptibility patterns of integron-positive and -negative isolates are shown in Table 3. All integron-positive isolates were resistant to cephalosporins, and the most effective antibiotic against these isolates was colistin. No significant correlation was observed between the presence of integrons and antibiotic resistance frequency, except for tetracycline with p = 0.025.
Table 3.
Relationship between antibiotic susceptibility pattern with integron and biofilm formation in A.baumannii isolates.
| Antibiotic | Integron-positive isolatesN (%) |
Integron-negative isolates N (%) |
p value |
Strong
biofilm-producing isolates N (%) |
Biofilm-negative isolates N (%) |
p value |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | I | S | R | I | S | R | I | S | R | I | S | |||||||||
| Tigecycline | 29 (47.5) | 0 (0) | 32 (52.45) | 3 (33.3) | 0 (0) | 6 (66.6) | 0.494 | 25 (45.45) | 0 (0) | 30 (54.54) | 1(20) | 0 (0) | 4 (80) | 0.377 | ||||||
| Colistin | 2 (3.27) | 0 (0) | 59 (96.7) | 0 (0) | 0 (0) | 9 (100) | 1 | 2 (3.63) | 0 (0) | 53 (96.36) | 0 (0) | 0 (0) | 5 (100) | 1 | ||||||
| Meropenem | 45(73.77) | 0 (0) | 16 (26.22) | 7 (77.7) | 0 (0) | 2 (22.2) | 1 | 40 (72.7) | 0 (0) | 15 (27.27) | 5 (100) | 0 (0) | 0 (0) | 0.318 | ||||||
| Imipenem | 43(70.5) | 3 (4.9) | 15 (24.59) | 7(77.7) | 0 (0) | 2(22.2) | 1 | 39 (70.9) | 3 (5.45) | 13 (23.6) | 4 (80) | 0 (0) | 1 (20) | 1 | ||||||
| Ampicillin-sulbactam | 47 (77) | 0 (0) | 14 (22.9) | 8 (88.8) | 0 (0) | 1 (11.1) | 0.672 | 41 (74.5) | 0 (0) | 14 (25.45) | 5 (100) | 0 (0) | 0 (0) | 1 | ||||||
| Tetracycline | 50 (81.9) | 0 (0) | 11 (18) | 4 (44.4) | 0 (0) | 5 (55.5) | 0.025 | 46 (83.6) | 0 (0) | 9 (16.36) | 3 (60) | 0 (0) | 2 (20) | 0.224 | ||||||
| Minocycline | 40 (65.57) | 0 (0) | 21 (34.4) | 3(33.3) | 0 (0) | 6 (66.6) | 0.079 | 37 (67.27) | 0 (0) | 18 (32.7) | 2 (40) | 0 (0) | 3 (60) | 0.337 | ||||||
| Ceftazidime | 61 (100) | 0 (0) | 0 (0) | 9 (100) | 0 (0) | 0 (0) | - | 55 (100) | 0 (0) | 0 (0) | 5 (100) | 0 (0) | 0 (0) | - | ||||||
| Cefepime | 61(100) | 0 (0) | 0 (0) | 9 (100) | 0 (0) | 0 (0) | - | 55 (100) | 0 (0) | 0 (0) | 5 (100) | 0 (0) | 0 (0) | - | ||||||
| Piperacillin | 59 (96.7) | 0 (0) | 2 (3.27) | 9 (100) | 0 (0) | 0 (0) | 1 | 54 (98.1) | 0 (0) | 1 (1.8) | 4 (80) | 0 (0) | 1 (20) | 0.169 | ||||||
| Cefotaxime | 61(100) | 0 (0) | 0 (0) | 9 (100) | 0 (0) | 0 (0) | - | 55 (100) | 0 (0) | 0 (0) | 5 (100) | 0 (0) | 0 (0) | - | ||||||
| Trimethoprim-sulfamethoxazole | 58 (95) | 0 (0) | 3 (5) | 9 (100) | 0 (0) | 0 (0) | 1 | 53 (96.36) | 0 (0) | 2 (3.6) | 5 (100) | 0 (0) | 0 (0) | 1 | ||||||
| Amikacin | 52 (85.2) | 2 (3.27) | 6 (9.8) | 7 (77.7) | 0 (0) | 2 (22.2) | 0.465 | 45 (81.8) | 2 (3.6) | 8 (14.5) | 4 (80) | 0 (0) | 1 (20) | 1 | ||||||
| Ciprofloxacin | 57 (93.4) | 0 (0) | 4 (6.55) | 8 (88.8) | 0 (0) | 1 (11.1) | 0.508 | 50 (90.9) | 0 (0) | 5 (9.09) | 5 (100) | 0 (0) | 0 (0) | 1 | ||||||
| Gentamicin | 49 (80.3) | 2 (3.27) | 10 (16.39) | 9 (100) | 0 (0) | 0 (0) | 0.497 | 46 (83.6) | 1 (1.8) | 8 (14.5) | 4 (80) | 0 (0) | 1 (20) | 0.612 | ||||||
| Tobramycin | 44 (72.1) | 3 (4.9) | 14 (22.9) | 8 (88.8) | 1 (11.1) | 0 (0) | 0.193 | 41 (74.5) | 2 (3.6) | 12 (21.8) | 3 (60) | 1 (20) | 1 (20) | 0.289 | ||||||
| Levofloxacin | 56 (91.8) | 0 (0) | 5 (8.19) | 9 (100) | 0 (0) | 0 (0) | 1 | 50 (90.9) | 0 (0) | 5 (9.09) | 5 (100) | 0 (0) | 0 (0) | 1 | ||||||
R: resistant; I: intermaediate; S: susceptible
Biofilm formation assay
Evaluation of biofilm formation was performed using the MTP method. Among all the isolates, 5 (7.1%) were non-biofilm producers, while the numbers of weak, moderate and strong biofilm producers were 3 (4.3%), 7 (10%), and 55 (78.5%), respectively. The OD570 values for positive and negative controls were 0.411 ± 0.041 and 0.071 ± 0.010, respectively. The proportion of strong biofilm producers in various wards was 30 (96.8%) in ICU, as well as 6 (10.9%) in surgery, 5 (9%) in urology, 6 (11%) in infectious diseases, 4 (7.2%) in general, 2 (3.6%) in pediatric, and 2 (3.6%) in neurology units. Additionally, of 55 strong biofilm producers, 47 (85.45%) and 8 (14.54%) were MDR and XDR, respectively. All the weak and moderate biofilm producers were MDR phenotypes. Among strong biofilm-producing isolates, 20 and 14 were from tracheal aspirates and catheter specimens, respectively, and resistance to the entire antibiotic, except for tigecycline, colistin, and minocycline, was above 70% and was not statistically significant when compared to the biofilm-negative isolates (Table 3).
Molecular typing
The results of two multiplex PCRs indicated six different PCR-based groups (G1, G2, G4, G7, G10, and G15) among A. baumannii isolates. 37 (52.8%) and 8 (11.4%) isolates belonged to groups 1 (IC II) and 2 (IC I), respectively. Other isolates belonged to 4 IC variants PCR-based groups, including 11 (15.7%), 6 (8.5%), 5 (7.1%), and 3 (4.2%), and to G10, G4, G15 and G7, respectively. The frequency of antibiotic resistance in three epidemic lineages is shown in Table 4. Resistance to carbapenems, tetracycline, tobramycin, and levofloxacin in IC II isolates was higher than the IC I.
Table 4.
Frequency of antibiotic resistance in three epidemic lineages resistant A. baumannii isolates.
| Antibiotic
resistance No(%) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC (No.) | IMP | MER | CIP | AMK | SXT | CFT | COL | AMP/S | TGC | PIP | CFP | CFZ | TET | MIN | LEV | TOB | GEN |
| IC I (8) | 4 (50) | 5 (62.5) | 8 (100) | 8 (100) | 8 (100) | 8 (100) | 1 (12.5) | 8 (100) | 4 (50) | 8 (100) | 8 (100) | 8 (100) | 5 (62.5) | 4 (50) | 7 (87.5) | 5 (62.5) | 7 (87.5) |
| IC II (37) | 26 (70.2) | 29 (78.3) | 34 (91.8) | 30 (81) | 37 (100) | 37 (100) | 1 (2.7) | 25 (67.5) | 16 (43.2) | 37 (100) | 37 (100) | 37 (100) | 30 (81) | 28 (75.6) | 34 (91.8) | 25 (67.5) | 30 (81) |
| IC 4 variants (25) | 20 (80) | 18 (72) | 23 (92) | 21 (84) | 22 (88) | 25 (100) | 0 (0) | 22 (88) | 12 (48) | 23 (92) | 25 (100) | 25 (100) | 19 (76) | 11 (44) | 24 (96) | 22 (88) | 21 (84) |
| Total (70) | 50 (71.4) | 52 (74.2) | 65 (92.8) | 59 (84.2) | 67 (95.7) | 70 (100) | 2 (2.8) | 55 (78.6) | 32 (45.7) | 68 (97.1) | 70 (100) | 70 (100) | 54 (77.1) | 43 (61.4) | 65 (92.8) | 52 (74.3) | 58 (82.8) |
GEN: gentamicin; TOB: tobramycin; LEV: levofloxacin; TET: tetracycline; MIN: minocycline; CTZ: ceftazidime; CFP: cefepime; PIP: piperacillin; TGC: tigecycline; Amp/S: ampicillin/Sulbactam; COL: colistin; CTX: cefotaxime; SXT: trimethoprim-sulphamethoxazole; AMK: amikacin; CIP: ciprofloxacin; IMI: imipenem; MEM: meropenem; R: resistant; I: intermaediate; S: susceptible; International clones: IC
Of 37 isolates belonging to IC II, 34 were biofilm producers; in addition, 17 (45.9%) were obtained from the ICU. Moreover, 4 isolates had blaOXA-23-like/blaOXA-24-like genes, 3 isolates possessed blaOXA-23-like/blaOXA-58-like genes, and 2 isolates had blaOXA-23-like/blaOXA-24-like/blaOXA-58-like genes. Among 37 isolates, 33 (89.2%) and 4 (10.8%) were MDR and XDR phenotypes, respectively.
Of 8 isolates belonging to IC I, 6 isolates were MDR phenotypes with strong biofilm formation capacity and class I integron. Additionally, of 11 isolates belonging to group 10, 8 isolates had blaOXA-23-like, 2 isolates had blaOXA-23-like/blaOXA-58-like, 1 isolate had blaOXA-23-like/blaOXA-24-like genes, and 3 isolates possessed blaIMP/blaVIM gene. In this group, all the isolates were resistant to imipenem, tobramycin, and levofloxacin.
Via the MLVA typing method, 70 A. baumannii isolates were grouped into 12 distinct MLVA types (MTs) with 5 clusters and 4 singleton genotypes. MLVA-AB_2396 showed a high level of diversity, whereas other MLVA allelic profiles indicated low diversity (Figure 1). The most common MTs were types III (20), IV (14), and XI (14). Among the isolates belonging to MT type III, 18 (90%) isolates had class I or II integron or both, and 16 (80%) isolates formed strong biofilm. Furthermore, all the 70 (100%) isolates were susceptible to colistin, and 8 (40%) isolates were from the ICU.
Figure 1.
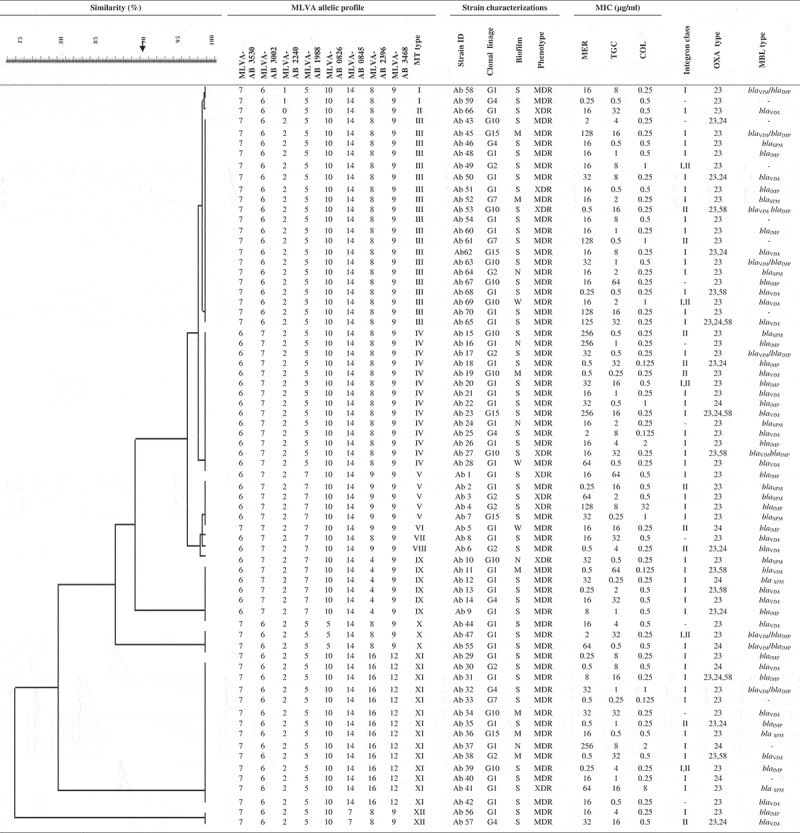
MLVA-8 allelic profiles and characteristics of A. baumannii strains. Cut-off value of 90% similarity.
Discussion
Over the past years, the antibiotic resistance of A. baumannii has dramatically increased, and like many other geographical regions, our country (Iran) has been confronted with this major problem [28]. In this study, the results of Vitek-2 system regarding the resistance patterns of 70 A. baumannii clinical isolates against 17 antibiotics revealed that 87.1% and 12.8% of the isolates were MDR and XDR phenotypes, respectively.
So far, several studies have reported different frequencies of MDR and XDR A. baumannii isolates. In a study by Maspi et al. [29] in Tehran, the capital of Iran, the frequency rate of MDR and XDR A. baumannii clinical isolates were determined as 94.2% and 71.2%, respectively, which are higher than the rates reported in the current study. In another investigation in Bushehr City, in the south of Iran, Rahimi et al. [30] observed a frequency rate of 21% for MDR and 41% for XDR A. baumannii isolates. The discrepancies between these results likely come from the geographical differences, the diverse origin of bacterial isolates, and the lack of a standard MDR/XDR pattern definition template in various studies.
Our results suggested that the third- and the fourth-generation cephalosporins had no effect on A. baumannii isolates. The study of El-Mahdy et al. [31] conducted in Saudi Arabia reflected a 100% resistance rate against the aforementioned antibiotics, which was comparable with our finding. On the other hand, our study revealed a higher resistance rate of A. baumannii isolates against the third- and the fourth-generation cephalosporins compared to previous studies in Serbia [32], India [33], and Poland [34].
The results of antibiotic susceptibility testing disclosed that more than half of the A. baumannii isolates were resistant to the aminoglycoside family, among which amikacin with 84.2% resistance rate had the least effect. These observations were in line with that of Upadhyay et al. [35] who reported a high-level of aminoglycoside resistance (79.2%) in A. baumannii isolates recovered from patients admitted to the ICU of a tertiary referral hospital in Northeastern India. Besides, in some regions of Iran, high-level aminoglycoside-resistant A. baumannii clinical isolates (more than 70%) were reported by Mirnejad et al. [36] and Hatami Moghadam et al. [37] whose results were in agreement with the findings of the current study.
More than 70% of our A. baumannii isolates were resistant to carbapenems, which is in accordance with the findings obtained by Maspi et al. [29] in Tehran (Iran), Rynga et al. [38] in New Delhi (India), and Nowak et al. [39] who recorded high resistance rates for imipenem (96.9%) and meropenem (87.7%) in A. baumannii isolates collected from patients with ventilator-associated pneumonia in Greece, Italy, and Spain. However, reports from Poland and Japan displayed considerable lower resistance rates for carbapenems, which contradicts our finding that more than 50% of the isolates were susceptible to carbapenems [34,40].
The emergence of MDR and XDR carbapenem-resistant clones harboring oxacillinases and metallo-β-lactamases genes has raised considerable concerns for health systems worldwide. Here, the most prevalent oxacillinase was the blaOXA-23-like (90%), a result similar to that of a former survey in which the blaOXA-23-like was recognized as the most detected gene [32]. This observation was also in agreement with earlier reports from Iran and Mediterranean countries like Turkey that highlighted the blaOXA-23-like gene as the most prevalent acquired oxacillinase in A. baumannii isolates [32,41]. The occurrence rate of 25.7% of blaOXA-24-like gene in the present investigation was in contrast to the rate obtained in the study of Castilho et al. [42] who did not observe this gene in any CRAB isolates from Brazil. Another study from Tehran, Iran, showed a lower incidence rate of blaOXA-24-like (1%) compared to the current research in which 11.4% of isolates harbored blaOXA-58-like gene, while blaOXA-143-like gene was not found in any isolates [43]. In agreement with our results, Sarikhani et al.’s [41] investigation from Qom, in north-central Iran, presented an occurrence rate of 55.6% and 14.4% for blaOXA-58-like and blaOXA-143-like genes, respectively. Until now, the emergence of new class D β-lactamases, blaOXA-143-like and blaOXA-235-like, in A. baumannii isolates was limited to Brazil, Mexico, and USA [44].
The metallo-β-lactamase genes were detected in 87.1% (61/70) of isolates. This finding in our study is supported by Soltani et al. [45] who reported the presence of 82.6% of these genes in A. baumannii isolates in the south of Iran. In the present research, 12 (17.1%), 18 (25.7%), and 22 (31.4%) isolates carried blaSPM, blaIMP, and blaVIM genes, respectively, while no isolates were positive for the blaNDM and blaSIM determinants. Maspi et al. [29] pointed out that 2.3%, 15.1%, 2.3%, 4.7%, and 2.3% of isolates carried blaVIM, blaIMP, blaSPM, blaGIM and blaSIM genes, respectively, which differs from our study. On the other hand, their results demonstrated that none of the tested strains harbored the blaNDM gene. In the studies conducted by Amin et al. [46] and Gholami et al. [47] in Iran, the prevalence of blaSPM gene was reported to be 5.19% and 3.63%, respectively. Pournajaf et al.’s [48] report from Iran and Gomma et al.’s [23] report from Egypt affirmed our finding that the blaVIM is the most prevalent metallo-β-lactamase.
Our results are in accord with several previous studies showing that colistin and tigecycline are still among the most effective drugs in the treatment of MDR and XDR A. baumannii [32,33,43]. The resistance rate of 2.8% for colistin in this investigation was comparable with its global pooled prevalence of 0.0–3.7, while the resistance rate of 45.7% for tigecycline was higher than its global pooled prevalence of 2.3–25.8 [49]. Likewise, Salehi et al. [43] observed a higher resistance proportion (77%) for tigecycline than global pooled prevalence among clinical A. baumannii isolates.
The ability of A. baumannii to colonize and produce biofilm on biotic and abiotic surfaces is one of the main bases of chronic and nosocomial infections caused by this bacterium [50]. In our study, the results of MTP method revealed the capability of biofilm production by 92.8% of isolates, among which 78.5% were strong biofilm producers. Based on earlier evidence from Iran and Korea, the same as this study, more than 70% of isolates were able to produce biofilm [50,51]. Furthermore, the ability of biofilm production in A. baumannii isolates was not significantly correlated with resistance to antibiotics. Previously, Rahimi et al. [30] and Qi et al. [52] signified an inverse relationship between biofilm formation ability and antibiotic resistance or the acquisition of MDR/XDR phenotypes in A. baumannii isolates, while a study by Yang et al. [53] revealed that the MDR A. baumannii isolates provided a higher biofilm formation capacity.
PCR assay data indicated that 75.4% and 16.3% of isolates harbored class I and class II integrons, respectively, while class III integron was not identified in any isolates. Halaji et al. [54] reported a higher prevalence in Isfahan, Iran, for class II integron (78.2%) than class I (63.9%) in clinical A. baumannii isolates, which contradicts our results. Remarkably, a number of studies in this area expressed a higher prevalence for class I than class II integrons, but they could not detect class III integrons [54,55]. The lack of correlation between the presence of integrons and antibiotic resistance, except for the tetracycline, was another finding of our study. However, some previous investigations from different regions of the world emphasized that A. baumannii isolates harboring integrons had more potential to become MDR than integron-negative isolates [54–56].
The three-locus dual assay multiplex PCR and MLVA-8 were performed in this study to assess the clonality of A. baumannii isolates. The results of three-locus multiplex PCR suggested six different PCR-based or IC lineages (G1, G2, G4, G7, G10, and G15) among A. baumannii isolates, while Farshadzadeh et al. [57] reported eight different IC types. The G1 (IC II) with a frequency of 52.8% was the most predominant group in this study, which correlates strongly with the finding that the IC II is the most frequent lineage in the world [58,59]. The incidence of group G2 (CI I; 11.4%) was lower than the frequency (19%) recorded for this IC lineage [60]. In the current study, 35.8% (25/70) of isolates belonged to IC variants, which was lower than the rate reported by Bahador et al. [60] who indicated the occurrence rate of 43% for this lineage. Based on the results of MLVA-8, A. baumannii isolates were assigned to 12 different MTs, each had 1–20 members with the similarity cut-off of 90%. However, in another study in Iran, 32 MT variants were reported [60]. In our study, the MLVA-AB_2396 had the highest level of diversity, whereas MLVA-AB_0845 had the highest level of diversity in Iran and Spain [61,62].
In another study from France, Hauck et al. [56] reported the efficiency of an automatized MLVA-10 assay for the rapid and efficient genotyping of A. baumannii isolates. In our study, the MLVA-AB_2240 had an amplification failure for one isolate (Ab 66), while in other investigations in Iran [63] and France [56], Abaum3468, Abaum3002, Abaum3530, and Abaum0826 had an amplification failure, which is likely due to the partial deletion of the locus. Recently, Graña-Miraglia et al. [64] have shown that distantly related lineages can coexist in the same hospital in Honduras and Mexico. Our study supports the same pattern as diverse MDR A. baumannii populations are co-circulating in the Southwest of Iran, including the Imam Khomeini Hospital in Ahvaz; importantly, this pattern makes it difficult to properly implement infection control policies. In this research, due to financial limitations, the sequencing of the MBL genes was not performed.
Conclusion
This study documented a high distribution rate of MDR A. baumannii isolates, harboring different metallo-β-lactamase and oxacillinase genes, in one of the main regions in the southwest of Iran. Significant resistance to most antibiotics, except for colistin and tigecycline, in A. baumannii isolates seems to pose a serious challenge to healthcare systems in the future. The diverse A. baumannii lineages in this study highlights the need for designing appropriate antibiotic surveillance programs in each region based on the predominant circulating clone in order to choose the best therapy and manage infection control policies.
Acknowledgments
This study was a part of the Ph.D. thesis of Saeed Khoshnood. We would like to thank the Department of Microbiology, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, for their cooperation. Our appreciation goes to the Vice Chancellor for Research affairs, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran, and Tropical and Infectious Diseases Research Center of the University for their financial (Grant No. OG-9739) and executive support.
Disclosure statement
The authors declare that they have no conflict of interest in this study.
References
- [1].Noori M, Karimi A, Fallah F, et al. High prevalence of metallo-beta-lactamase producing Acinetobacter baumannii isolated from two hospitals of Tehran, Iran. Arch Ped Infect Dis. 2014;2:3. [Google Scholar]
- [2].Neonakis IK, Spandidos DA, Petinaki E.. Confronting multidrug-resistant Acinetobacter baumannii: a review. Int J Antimicrob Agents. 2011;37(2):102–109. [DOI] [PubMed] [Google Scholar]
- [3].Hassan Nejad N, Bahador A, Hayati Rudbari N, et al. Comparison of ompA gene-targeted real-time PCR with the conventional culture method for detection of Acinetobacter baumanii in pneumonic BALB/c mice. Iran Biomed J. 2019;23(2):159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Batarseh A, Al-Sarhan A, Maayteh M, et al. Antibiogram of multidrug resistant Acinetobacter baumannii isolated from clinical specimens at King Hussein medical centre, Jordan: a retrospective analysis. East Mediterr Health J. 2015;21(11):11. [DOI] [PubMed] [Google Scholar]
- [5].Rezaei E, Safari H, Naderinasab M, et al. Common pathogens in burn wound and changes in their drug sensitivity. Burns. 2011;37(5):805–807. [DOI] [PubMed] [Google Scholar]
- [6].Higgins PG, Dammhayn C, Hackel M, et al. Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2010;65(2):233–238. [DOI] [PubMed] [Google Scholar]
- [7].Josheghani SB, Moniri R, Firoozeh F, et al. Emergence of bla OXA-carrying carbapenem resistance in multidrug-resistant Acinetobacter baumannii in the intensive care unit. Iran Red Crescent Med J. 2017;19:5. [Google Scholar]
- [8].Tchuinte PL, Rabenandrasana MA, Kowalewicz C, et al. Phenotypic and molecular characterisations of carbapenem-resistant Acinetobacter baumannii strains isolated in Madagascar. Antimicrob Resist Infect Control. 2019;8(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hujer KM, Hujer AM, Endimiani A, et al. Rapid determination of quinolone resistance in Acinetobacter spp. J Clin Microbiol. 2009;47(5):1436–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Villalon P, Valdezate S, Medina-Pascual MJ, et al. Clonal diversity of nosocomial epidemic Acinetobacter baumannii strains isolated in Spain. J Clin Microbiol. 2011;49(3):875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Singhai M, Malik A, Shahid M, et al. A study on device-related infections with special reference to biofilm production and antibiotic resistance. J Glob Infect Dis. 2012;4(4):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Diancourt L, Passet V, Nemec A, et al. The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS One. 2010;4:e10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pourcel C, Minandri F, Hauck Y, et al. Identification of variable-number tandem-repeat (VNTR) sequences in Acinetobacter baumannii and interlaboratory validation of an optimized multiple-locus VNTR analysis typing scheme. J Clin Microbiol. 2011;49(2):539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Graña-Miraglia L, Lozano LF, Velázquez C, et al. Rapid gene turnover as a significant source of genetic variation in a recently seeded population of a healthcare-associated pathogen. Front Microbiol. 2017;8:1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Murray PR, Baron EJ, Jorgensen JH, et al. Manual of clinical microbiology. Washington DC: American Society for Microbiology; 2006. [Google Scholar]
- [16].Higgins PG, Wisplinghoff H, Krut O, et al. A PCR-based method to differentiate between Acinetobacter baumannii and Acinetobacter genomic species 13TU. Clin Microbiol Infect. 2007;13(12):1199–1201. [DOI] [PubMed] [Google Scholar]
- [17].CLSI. M100-S28 . Performance standards for antimicrobial susceptibility testing; Twenty-eight informational supplement; 2018.
- [18].Jones RN, Ferraro MJ, Reller LB, et al. Multicenter studies of tigecycline disk diffusion susceptibility results for Acinetobacter spp. J Clin Microbiol. 2007;45(1):227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Manchanda V, Sanchaita S, Singh N.. Multidrug resistant Acinetobacter. J Glob Infect Dis. 2010;2(3):291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yong D, Lee K, Yum JH, et al. Imipenem-EDTA disk method for differentiation of metallo-betalactamase-producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2002;40(10):3798–3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Higgins PG, Pérez-Llarena FJ, Zander E, et al. OXA-235, a novel class D β-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob Agent Chemother. 2013;57(5):2121–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fallah F, Noori M, Hashemi A, et al. Prevalence of blaNDM, blaPER, blaVEB, blaIMP, and blaVIM genes among Acinetobacter baumannii isolated from two hospitals of Tehran, Iran. Scientifica (Cairo). 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gomaa F, Helal Z, Khan M. High prevalence of blaNDM-1, blaVIM, qacE, and qacEΔ1 genes and their association with decreased susceptibility to antibiotics and common hospital biocides in clinical isolates of Acinetobacter baumannii. Microorganisms. 2017;5(2):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dillon B, Thomas L, Mohmand G, et al. Multiplex PCR for screening of integrons in bacterial lysates. J Microbiol Methods. 2005;62(2):221–232. [DOI] [PubMed] [Google Scholar]
- [25].O’Toole GA. Microtiter dish biofilm formation assay. JoVE (J Vis Exp). 2011(47):e2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang D, Xia J, Xu Y, et al. Biological features of biofilm-forming ability of Acinetobacter baumannii strains derived from 121 elderly patients with hospital-acquired pneumonia. Clin Exp Med. 2016;16(1):73–80. [DOI] [PubMed] [Google Scholar]
- [27].Turton JF, Gabriel SN, Valderrey C, et al. Use of sequence-based typing and multiplex PCR to identify clonal lineages of outbreak strains of Acinetobacter baumannii. Clin Microbiol Infect. 2007;13(8):807–815. [DOI] [PubMed] [Google Scholar]
- [28].Pourhajibagher M, Hashemi FB, Pourakbari B, et al. Antimicrobial resistance of Acinetobacter baumannii to imipenem in Iran: a systematic review and meta-analysis. Open Microbiol J. 2016;10(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Maspi H, Hosseini HM, Amin M, et al. High prevalence of extensively drug-resistant and metallo beta-lactamase-producing clinical Acinetobacter baumannii in Iran. Microb Pathog. 2016;98:155–159. [DOI] [PubMed] [Google Scholar]
- [30].Rahimi S, Farshadzadeh Z, Taheri B, et al. The relationship between antibiotic resistance phenotypes and biofilm formation capacity in clinical isolates of Acinetobacter baumannii. Jundishapur J Microbiol. 2018;11(8):e74315. [Google Scholar]
- [31].El-Mahdy TS, Al-Agamy MH, Al-Qahtani AA, et al. Detection of blaOXA-23-like and blaNDM-1 in Acinetobacter baumannii from the eastern region, Saudi Arabia. Microb Drug Resist. 2017;23(1):115–121. [DOI] [PubMed] [Google Scholar]
- [32].Boral B, Ö U, Ergin A, et al. Acinetobacter study group. A prospective multicenter study on the evaluation of antimicrobial resistance and molecular epidemiology of multidrug-resistant Acinetobacter baumannii infections in intensive care units with clinical and environmental features. Ann Clin Microbiol Antimicrob. 2019;18(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tewari R, Chopra D, Wazahat R, et al. Antimicrobial susceptibility patterns of an emerging multidrug resistant nosocomial pathogen: Acinetobacter baumannii. Malays J Med Sci. 2018;25(3):129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Grochowalska A, Kozioł-Montewka M, Sobieszczańska A. Analysis of Acinetobacter baumannii resistance patterns in patients with chronic obstructive pulmonary disease (COPD) in terms of choice of effective empiric antibiotic therapy. Ann Agric Environ Med. 2017;24(2):307–311. [DOI] [PubMed] [Google Scholar]
- [35].Upadhyay S, Khyriem AB, Bhattacharya P, et al. High-level aminoglycoside resistance in Acinetobacter baumannii recovered from Intensive Care Unit patients in Northeastern India. Indian J Med Microbiol. 2018;36(1):43–48. [DOI] [PubMed] [Google Scholar]
- [36].Mirnejad R, Heidary M, Bahramian A, et al. Evaluation of polymyxin B susceptibility profile and detection of drug resistance genes among Acinetobacter Baumannii clinical isolates in Tehran, Iran during 2015–2016. Mediterr J Hematol Infect Dis. 2018;10(1):e2018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hatami Moghadam R, Alvandi AH, Akbari N, et al. The frequency of multidrug-resistance and extensively drug-resistant Acinetobacter baumannii in West of Iran. J Res Med Dent Sci. 2018;6(2):112–119. [Google Scholar]
- [38].Rynga D, Shariff M, Deb M. Phenotypic and molecular characterization of clinical isolates of Acinetobacter baumannii isolated from Delhi, India. Ann Clin Microbiol Antimicrob. 2015;14(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nowak J, Zander E, Stefanik D, et al. High incidence of pandrug-resistant Acinetobacter baumannii isolates collected from patients with ventilator-associated pneumonia in Greece, Italy and Spain as part of the magic bullet clinical trial. J Antimicrob Chemother. 2017;72(12):3277–3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fujikura Y, Yuki A, Hamamoto T, et al. Blood stream infections caused by Acinetobacter baumannii group in Japan–epidemiological and clinical investigation. J Infect Chemother. 2016;22(6):366–371. [DOI] [PubMed] [Google Scholar]
- [41].Sarikhani Z, Nazari R, Rostami MN. First report of OXA-143-lactamase producing Acinetobacter baumannii in Qom, Iran. Iran J Basic Med Sci. 2017;20(11):1282–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Castilho SRA, Godoy CSM, Guilarde AO, et al. Acinetobacter baumannii strains isolated from patients in intensive care units in Goiânia, Brazil: molecular and drug susceptibility profiles. PLoS One. 2017;12(5):e0176790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Salehi B, Goudarzi H, Nikmanesh B, et al. Emergence and characterization of nosocomial multidrug-resistant and extensively drug-resistant Acinetobacter baumannii isolates in Tehran, Iran. J Inf Chemotherapy. 2018;24(7):515–523. [DOI] [PubMed] [Google Scholar]
- [44].Joshi PR, Acharya M, Kakshapati T, et al. Co-existence of blaOXA-23 and blaNDM-1 genes of Acinetobacter baumannii isolated from Nepal: antimicrobial resistance and clinical significance. Antimicrob Resist Infect Control. 2017;6(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Soltani B, Heidari H, Ebrahim-Saraie HS, et al. Molecular characteristics of multiple and extensive drug-resistant Acinetobacter baumannii isolates obtained from hospitalized patients in Southwestern Iran. Infez Med. 2018;26(1):67–76. [PubMed] [Google Scholar]
- [46].Amin M, Navidifar T, Shooshtari FS, et al. Association of the genes encoding Metallo-β-Lactamase with the presence of integrons among multidrug-resistant clinical isolates of Acinetobacter baumannii. Infect Drug Resist. 2019;12:1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gholami M, Moshiri M, Ahanjan M, et al. The diversity of class B and class D carbapenemases in clinical Acinetobacter baumannii isolates. Infez Med. 2018;26(4):329–335. [PubMed] [Google Scholar]
- [48].Pournajaf A, Rajabnia R, Razavi S, et al. Molecular characterization of carbapenem-resistant Acinetobacter baumannii isolated from pediatric burns patients in an Iranian hospital. Trop J Pharm Res. 2018;17(1):135–141. [Google Scholar]
- [49].Xie R, Zhang XD, Zhao Q, et al. Analysis of global prevalence of antibiotic resistance in Acinetobacter baumannii infections disclosed a faster increase in OECD countries. Emerg Microbes Infect. 2018;7(1):31. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zeighami H, Valadkhani F, Shapouri R, et al. Virulence characteristics of multidrug resistant biofilm forming Acinetobacter baumannii isolated from intensive care unit patients. BMC Infect Dis. 2019;19(1):629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Youn Sung J. Molecular characterization and antimicrobial susceptibility of biofilm-forming Acinetobacter baumannii clinical isolates from Daejeon, Korea. Korean J Clin Lab Sci. 2018;50(2):100–109. [Google Scholar]
- [52].Qi L, Li H, Zhang C, et al. Relationship between antibiotic resistance, biofilm formation, and biofilm specific resistance in Acinetobacter baumannii. Front Microbiol. 2016;7:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yang CH, Su PW, Moi SH, et al. Biofilm formation in Acinetobacter baumannii: genotype-phenotype correlation. Molecules. 2019;24(10):1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Halaji M, Rezaei A, Zalipoor M, et al. Investigation of class I, II, and III integrons among Acinetobacter baumannii isolates from hospitalized patients in Isfahan, Iran. Oman Med J. 2018;33(1):37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chen J, Li H, Yang J, et al. Prevalence and characterization of integrons in multidrug resistant Acinetobacter baumannii in Eastern China: a multiple-hospital study. Int J Environ Res Public Health. 2015;12(8):10093–100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hauck Y, Soler C, Jault P, et al. Diversity of Acinetobacter baumannii in four French military hospitals, as assessed by multiple locus variable number of tandem repeats analysis. PLoS One. 2012;7(9):e44597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Farshadzadeh Z, Hashemi FB, Rahimi S, et al. Wide distribution of carbapenem resistant Acinetobacter baumannii in burns patients in Iran. Front Microbiol. 2015;6:1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Liu CC, Tang CY, Chang KC, et al. A comparative study of class 1 integrons in Acinetobacter baumannii. Gene. 2014;544(1):75–82. [DOI] [PubMed] [Google Scholar]
- [59].Martins N, Dalla-Costa L, Uehara AA, et al. Emergence of Acinetobacter baumannii international clone II in Brazil: reflection of a global expansion. Infect Genet Evol. 2013;20:378–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bahador A, Farshadzadeh Z, Raoofian R, et al. Association of virulence gene expression with colistin-resistance in Acinetobacter baumannii: analysis of genotype, antimicrobial susceptibility, and biofilm formation. Ann Clin Microbiol Antimicrob. 2018;17(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Najar-Peerayeh S, Karamostaji A. Evaluation of multilocus variable-number tandem-repeat (MLVA-8 Orsay) for typing of carbapenem-resistant Acinetobacter baumannii isolated from patients in Tehran, Iran. Arch Clin Infect Dis. 2019;14(1):e64402. [Google Scholar]
- [62].Villalón P, Valdezate S, Cabezas T, et al. Endemic and epidemic Acinetobacter baumannii clones: A twelve-year study in a tertiary care hospital. BMC Microbiol. 2015;15(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Peerayeh SN, Karmostaji A. Molecular identification of resistance determinants, integrons and genetic relatedness of extensively drug resistant Acinetobacter baumannii isolated from hospitals in Tehran, Iran. Jundishapur J Microbiol. 2015;8(7):e27021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Graña-Miraglia L, Evans BA, López-Jácome LE, et al. Origin of OXA-23 variant OXA-239 from a recently emerged lineage of Acinetobacter baumannii international clone V. mSphere. 2020;26(5):1. [DOI] [PMC free article] [PubMed] [Google Scholar]


