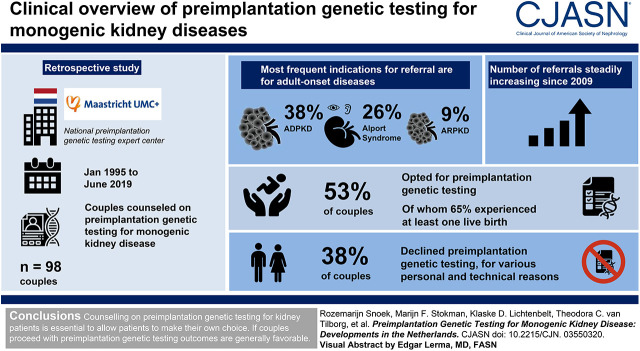
Visual Abstract
Keywords: genetic renal disease, ADPKD, Alport syndrome, Genetic Testing, Kidney Diseases
Abstract
Background and objectives
A genetic cause can be identified for an increasing number of pediatric and adult-onset kidney diseases. Preimplantation genetic testing (formerly known as preimplantation genetic diagnostics) is a reproductive technology that helps prospective parents to prevent passing on (a) disease-causing mutation(s) to their offspring. Here, we provide a clinical overview of 25 years of preimplantation genetic testing for monogenic kidney disease in The Netherlands.
Design, setting, participants, & measurements
This is a retrospective cohort study of couples counseled on preimplantation genetic testing for monogenic kidney disease in the national preimplantation genetic testing expert center (Maastricht University Medical Center+) from January 1995 to June 2019. Statistical analysis was performed through chi-squared tests.
Results
In total, 98 couples were counseled regarding preimplantation genetic testing, of whom 53% opted for preimplantation genetic testing. The most frequent indications for referral were autosomal dominant polycystic kidney disease (38%), Alport syndrome (26%), and autosomal recessive polycystic kidney disease (9%). Of couples with at least one preimplantation genetic testing cycle with oocyte retrieval, 65% experienced one or more live births of an unaffected child. Of couples counseled, 38% declined preimplantation genetic testing for various personal and technical reasons.
Conclusions
Referrals, including for adult-onset disease, have increased steadily over the past decade. Though some couples decline preimplantation genetic testing, in the couples who proceed with at least one preimplantation genetic testing cycle, almost two thirds experienced at least one live birth rate.
Introduction
CKD has an estimated global prevalence of 11%–13% and is associated with high morbidity and mortality (1–3). Recent studies have shown that a monogenic cause can be identified in 20%–40% of patients with childhood and adult-onset CKD (4–10).
Identifying the genetic cause in patients with monogenic kidney disease allows for counseling on prognosis and therapeutic options (11–13). Moreover, the risk of having affected children and the options regarding family planning can be discussed (5,10). Preconception counseling encompasses everything from expected pregnancy outcomes for mother and child to recurrence risk, invasive prenatal diagnosis, and preimplantation genetic testing (formerly known as preimplantation genetic diagnostics) (14). In The Netherlands, prospective parents can be counseled on the latter two options when, due to the severity of the kidney disease in the family, they might want to prevent the birth of an affected child.
Counseling on these options enables prospective parents to make a well informed decision regarding reproduction. When invasive prenatal diagnostic is permitted, chorionic villi sampling can be performed at 11–14 weeks or amniocentesis at 16 weeks of gestation (15). If genetic testing shows that the fetus is affected, the prospective parents have the option to terminate the pregnancy to prevent the birth of a child affected with monogenic kidney diseases.
Here, we focus on preimplantation genetic testing, which entails genetic testing of one or two cells derived from a six to eight cell–stage day 3 embryo or of five to ten trophectoderm cells derived from a blastocyst-stage embryo at day 5 or 6 after in vitro fertilization with intracytoplasmic sperm injection (16). Only embryos without the parental mutation(s) in the biopsied cell(s) are eligible for transfer into the uterus (16).
Preimplantation genetic testing was first performed in The Netherlands in 1995 and has been part of reimbursed medical care since 2008 (up to three cycles). The Maastricht University Medical Center+ (MUMC+) is the only center in The Netherlands licensed to perform preimplantation genetic testing. In the Dutch Preimplantation Genetic Testing The Netherlands (PGT The Netherlands) consortium, the MUMC+ collaborates with multidisciplinary in vitro fertilization teams in three tertiary center fertility clinics. In all four centers, women are treated, whereas the actual preimplantation genetic testing is performed in the laboratory of the MUMC+ (so-called “transport preimplantation genetic testing”).
To date, preimplantation genetic testing has been applied for over 500 conditions worldwide (16,17). Over the past 10–15 years, indications have shifted from diseases with pediatric onset and/or severe phenotype to adult-onset diseases and conditions with reduced penetrance, such as specific forms of hereditary cancer (18). Requests for preimplantation genetic testing in not previously requested genetic disorders are reviewed on the basis of general disease severity by a national multidisciplinary indication committee composed of clinical geneticists, gynecologists, medical ethicists, and patient representatives. Each individual couple seeking preimplantation genetic testing will also be reviewed by a separate committee that takes into account the disease severity, genotype-phenotype correlation, and the technical possibilities of not passing on the specific variant. The Dutch system of nationwide regulation and reimbursement is unique, and it has allowed The Netherlands to be one of the first and few countries where couples have the option to opt for preimplantation genetic testing for monogenic kidney disease, including adult-onset forms of kidney disease.
On preimplantation genetic testing for monogenic kidney disease specifically, there are few reports in literature. To date, 12 papers on preimplantation genetic testing for monogenic kidney disease have been published (Supplemental Material), mostly describing individual patients or small patient series and focusing on severe and early-onset diseases (19–30). In recent years, some reports have been published on larger series for specific diseases, such as the paper by Berckmoes et al. (21) on 43 couples who underwent preimplantation genetic testing for autosomal dominant polycystic kidney disease (ADPKD). However, there is limited information on uptake and success rates of preimplantation genetic testing for monogenic kidney disease in general.
In this paper, we show the developments in preimplantation genetic testing for monogenic kidney disease in the PGT The Netherlands consortium over the past 25 years, reviewing the indications, uptake, pregnancy rates, and parent-related factors. We illustrate advantages and pitfalls of preimplantation genetic testing for monogenic kidney disease and provide clinical recommendations for shared decision making with regard to preimplantation genetic testing for monogenic kidney disease.
Materials and Methods
Institutional review board approval was obtained in the MUMC+ to perform a retrospective cohort study, adhering to the Declaration of Helsinki. The cohort consisted of couples who were counseled in the MUMC+ on preimplantation genetic testing for monogenic kidney disease in the period from January 1, 1995 to June 1, 2019. Monogenic kidney disease was defined as a disorder with kidney disease as the main feature.
For all enrolled couples, data on baseline characteristics, genetic diagnosis, preimplantation genetic testing parameters, and, if applicable, reasons for declining preimplantation genetic testing were retrieved from the electronic patient files. Data were retrieved from the MUMC+ patient files only. Data on preimplantation genetic testing cycles could have only been included if a treatment cycle reached the stage of oocyte retrieval. Data were collected up to June 1, 2019. We collected data on all of the pregnancies that the couples had after they were first counseled on preimplantation genetic testing.
Several outcome parameters were defined: “ongoing pregnancy” was defined as a pregnancy >12 weeks of gestation, and “live birth” was defined as the birth of a child surviving >24 hours. Whether a child was affected or unaffected with the parental monogenic kidney disease was determined on the basis of either clinical diagnosis and/or genetic testing. If no information was available on the disease status of the child, it was noted as “unknown.” If age at onset is <18 years, the disease was defined as having a “pediatric onset,” and if age at onset is >18 years, it was defined as “adult onset.” Age at onset of disease was defined according to the approximate age at onset of CKD mentioned in literature (7).
Analyses of all data were performed with SPSS for Windows (version 25; IBM). The chi-squared tests were performed two sided, and the probability of a type 1 error was set at 0.05. All data are represented cumulatively per couple unless otherwise indicated.
Results
The cohort consists of 98 couples, of whom the baseline characteristics are presented in Table 1. The median CKD stage was stage 1, with 88% of affected prospective parents being in early CKD (stages 1–3). Nine affected prospective parents had received a kidney transplant, of whom eight were fathers. The majority of couples had autosomal dominant or X-linked disease, with the prospective mother more often being the affected parent than the father (detailed in Table 1).
Table 1.
Baseline characteristics
| Characteristic | Total Couples, n=98 |
|---|---|
| Maternal characteristics | |
| Maternal age at first counseling, yr, median (range) | 32 (22–40) |
| Nulliparity, n (%) | 63 (64) |
| Kidney disease characteristics | |
| Affected prospective parent CKD stage at counseling, median (range) | 1 (1–5) |
| Affected prospective parent CKD stage >3, n (%) | 12 (12) |
| Affected prospective parent post-transplantation, n (%) | 9 (9) |
| Genetic characteristics, n (%) | |
| Autosomal dominant disease | 53 (54) |
| Autosomal recessive disease | 18 (18) |
| X-linked disease | 27 (28) |
| In case of autosomal dominant or X-linked disease, n of total cohort (%) | |
| Genetically affected parent is the father | 33 (34) |
| Genetically affected parent is the mother | 47 (48) |
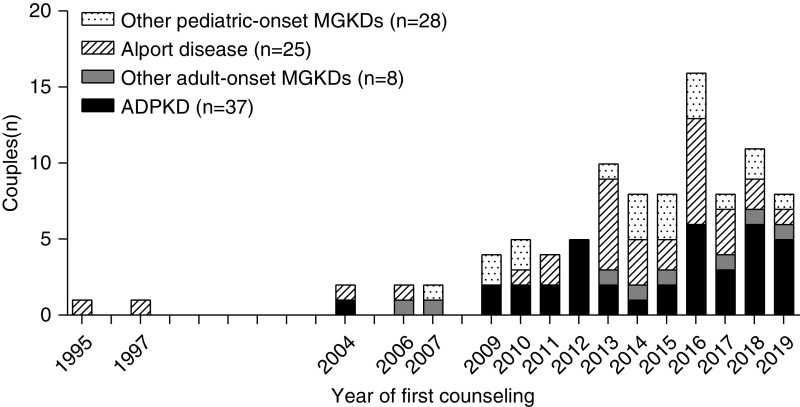
Initially, preimplantation genetic testing referrals for monogenic kidney disease were incidental, and indications predominantly concerned pediatric-onset kidney disease (Figure 1). The first couple with adult-onset disease (ADPKD) was referred in 2004. From 2009 onward, the number of referrals for adult-onset disease steadily increased and became the most frequent referral indication. ADPKD was the most frequent reason for referral (38%).
Figure 1.
The number of couples (n) counseled on preimplantation genetic testing for monogenic kidney disease (MGKD) in The Netherlands has steadily increased since the first referral in 1995, including for adult-onset diseases. ADPKD, autosomal dominant polycystic kidney disease.
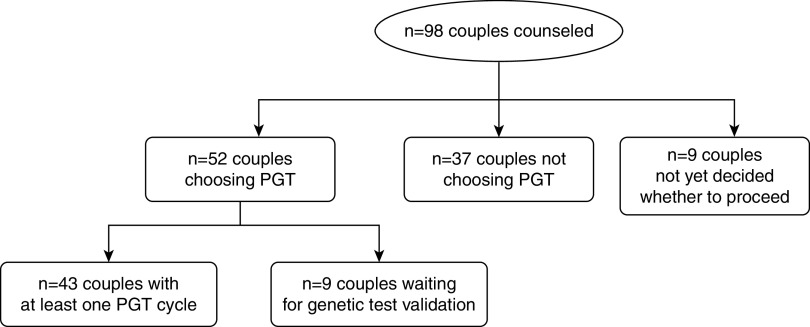
Of the 98 couples who were counseled in the MUMC+, 52 (53%) chose to proceed with preimplantation genetic testing; 43 (44% of total cohort) had undergone at least one cycle that reached the oocyte retrieval stage by June 1, 2019; and 9 (9% of total cohort) were waiting for the validation of the single-cell genetic test for their specific mutation (Figure 2, Tables 2 and 3). In those 43 couples, a total of 79 preimplantation genetic testing cycles reaching at least the stage of oocyte retrieval were performed, with a median of two cycles (interquartile range [IQR], 1) per couple. Cumulative results per couple show a median of ten embryos (IQR, 14) available for biopsy and a median of three embryos (range, 1–14) genetically unaffected. Sixty-five percent (n=28) of couples who had at least one cycle had at least one live birth through preimplantation genetic testing, with four couples having had more than one unaffected live birth through preimplantation genetic testing at the time of analysis. For those who became pregnant after preimplantation genetic testing, the median duration from counseling to the first live birth was 2 years (IQR, 1). There were five couples who had a spontaneous pregnancy after successful (n=2) or unsuccessful preimplantation genetic testing procedures (n=3).
Figure 2.
Flowchart of the n=98 couples who were counseled on preimplantation genetic testing (PGT) for monogenic kidney disease.
Table 2.
Referral indications on preimplantation genetic testing for monogenic kidney disease
| Disease | n (%) | Gene (n) |
|---|---|---|
| Autosomal dominant inheritance | ||
| ADPKDa | 37 (38) | PKD1 (35), PKD2 (2) |
| Brachio-oto-renal syndrome | 5 (5) | EYA1 (5) |
| INF2-related FSGSa | 3 (3) | INF2 (3) |
| Nail-patella syndromea | 3 (3) | LMX1B (3) |
| PAX2-related disease | 2 (2) | PAX2 (2) |
| aHUSa | 1 (1) | CFH (1) |
| ADTKD-HNF1Ba | 1 (1) | HNF1B (1) |
| Autosomal recessive inheritance | ||
| ARPKD | 9 (9) | PKHD1 (9) |
| Joubert syndrome | 7 (7) | CEP290 (5), TCTN3 (1), C5ORF42 (1) |
| Bardet–Biedl syndrome | 1 (1) | BBS7 (1) |
| Cystinosis | 1 (1) | CTNS (1) |
| Nephrotic syndrome | 1 (1) | COQ2 (1) |
| X-linked inheritance | ||
| Alport syndrome | 25 (26) | COL4A5 (25) |
| Nephrogenic diabetes insipidus | 2 (2) | AVPR2 (2) |
ADPKD, autosomal dominant polycystic kidney disease; aHUS, atypical hemolytic-uremic syndrome; ADTKD, autosomal dominant tubulointerstitial kidney disease; ARPKD, autosomal recessive polycystic kidney disease.
Adult-onset disease.
Table 3.
Preimplantation genetic testing and pregnancy outcomes
| Patient Category | n (%) | Median per Couple of Cumulative Cycles (Interquartile Range) |
|---|---|---|
| Couples with at least one preimplantation genetic testing cyclea | 43 (44) | |
| Cycles with oocyte retrieval | 79 | 2 (1) |
| Embryos for biopsy | 537 | 10 (14) |
| Of which embryos genetically unaffected and suitable for transferb | 190 (35) | 3 (5) |
| Couples with one or more pregnancy going >12-wk gestational age | 31 (72) | 1 (1) |
| Couples with one or more unaffected live births | 28 | |
| Couples with one unaffected live birth | 24 (56) | N/A |
| Couples with more than unaffected live birth | 4 (9) | N/A |
| Duration from referral to first live birth, yr | N/A | 2 (1) |
| Couples not proceeding with preimplantation genetic testing | 37 (38) | |
| Pregnancies without preimplantation genetic testing going >12-wk gestational age | 19 (49) | N/A |
| Terminations of pregnancy after diagnosis of affected fetus (either with fetal ultrasound or invasive prenatal diagnostics) | 3 (8) | N/A |
| Live birthsc | 15 (41) | N/A |
| Of which known to be affected | 4 (27) | N/A |
| Of which not affected | 4 (27) | |
| Of which affected status unknownd | 7 (47) | |
| Couples waiting for validation of the single-cell genetic test on June 1, 2019 | 9 (9) | N/A |
| Couples not yet decided on June 1, 2019 | 9 (9) | N/A |
N/A, not applicable.
Including couples not yet finished with the maximum of three reimbursed cycles on June 1, 2019.
In 2%–3%, embryo quality after thawing was too poor to perform the genetic test; thus, they were not suitable for testing and transfer.
One of 19 pregnancies going >12-wk gestational age resulted in a midtrimester loss.
Not all have received genetic testing due to the patient being a minor.
Thirty-seven couples (38%) chose not to proceed with preimplantation genetic testing after counseling for various reasons listed in Table 4. There were also nine couples (9%) who had not yet decided whether to proceed as of June 1, 2019 (Table 3). In couples not choosing preimplantation genetic testing, the main reasons were that (prospective) parents preferred conceiving spontaneously and performing invasive prenatal diagnostics (14%) or they did not want to wait for the time-consuming preimplantation genetic testing procedure and decided to accept the risk of having an affected child (11%). Five couples (14%) had a spontaneous pregnancy between the first and second counseling sessions. Not proceeding with preimplantation genetic testing was not independently influenced by maternal age (P=0.13), nor was it influenced by kidney disease–related variables, such as the CKD stage of the affected prospective (P=0.37) or the indication being adult-onset disease (P=0.11), or inheritance-related factors like disease inheritance pattern (P=0.64) or having an affected previous child (P=0.41).
Table 4.
Patient-reported reasons for declining preimplantation genetic testing after counseling
| Patient Category | n (%) |
|---|---|
| Choice by couple | 20 (54) |
| Declined without giving a specific reason | 9 (24) |
| Opt for spontaneous pregnancy with PND | 5 (14) |
| Couple does not want to wait for PGT | 4 (11) |
| Couple deems parental health too poor | 2 (5) |
| Technical reason | 9 (24) |
| Maternal age >42 yr at estimated time of start of IVF/PGT | 4 (11) |
| Single-cell genetic test not possible | 2 (5) |
| Specific indication is not allowed yet | 2 (5) |
| Ovarian reserve does not support IVF | 1 (3) |
| Other | 8 (22) |
| Spontaneous pregnancy prior to first PGT cycle | 5 (14) |
| Couple separated | 3 (8) |
PND, invasive prenatal genetic diagnostics; PGT, preimplantation genetic testing; IVF, in vitro fertilization.
Of the couples who did not proceed with preimplantation genetic testing, 18 had one spontaneous pregnancy (Table 3), resulting in 15 live births. Three pregnancies were terminated because invasive prenatal diagnostics showed the fetus to be affected with autosomal recessive polycystic kidney disease (n=1), Alport syndrome (n=1), or Joubert syndrome (n=1). Of the live births, four children (27%) were affected after couples chose not to have invasive prenatal diagnostics during the pregnancy (n=2 ADPKD, n=1 brachio-oto-renal syndrome, and n=1 nail-patella syndrome), and four children were genetically unaffected. In seven children from families with adult-onset disease, their disease status is unknown because genetic testing was not performed. Duration from counseling to live birth without preimplantation genetic testing was 2 years (range, 0–5).
Discussion
Over the past decade, preimplantation genetic testing has been increasingly performed in The Netherlands for couples to prevent passing monogenic kidney disease on to their offspring. There has been a strong increase in preimplantation genetic testing referrals for monogenic kidney disease since 2009. This can be explained on the one hand by increasing technical possibilities in preimplantation genetic testing, but also by an increase in referrals for adult-onset monogenic kidney disease, which reflects an increase in preimplantation genetic testing referrals for adult-onset disease in general (34). This underscores the potential influence of disease burden, whether that be pediatric or adult onset, on the choice for preimplantation genetic testing (30). The importance of disease burden was recognized by the national committee of clinical geneticists, gynecologists, medical ethicists, and patient representatives that decided on the allowed preimplantation genetic testing indications (35). Through consensus discussion, this committee moved to allow adult-onset disease indications in the early 2000s, incorporating them in the three-cycle insurance reimbursement scheme, thereby offering couples with adult-onset disease the option of preimplantation genetic testing (35). As the number of kidney diseases for which a monogenic cause can be identified continues to grow, it becomes increasingly important to timely counsel patients on their reproductive options in order to enable them to make an informed choice (10).
In general, reasons to proceed with preimplantation genetic testing depend on the nature and severity of the condition, the onset of symptoms, and the affected status of the parents and current children (36). Other factors in choosing preimplantation genetic testing include the wish to avoid suffering for offspring and feelings of guilt related to passing on the disease to future generations (37,38). Although reasons for choosing preimplantation genetic testing were not systematically recorded for our cohort, the relevance of the consideration to avoid the disorder in offspring is illustrated by the decision of three couples to terminate a pregnancy after prenatal testing had shown that the child was affected.
The 65% of couples who had at least one live birth through preimplantation genetic testing in our cohort is high. The PGT The Netherlands consortium reports that 32% of all started preimplantation genetic testing cycles resulted in a live birth (cumulative data since 1995) (35). However, a couple may start more than one cycle, and therefore, these data are difficult to compare because per couple pregnancy rates are not available for the total Dutch preimplantation genetic testing population (34). One should note that because we only include data from the MUMC+ preimplantation genetic testing procedure, we cannot report on maternal factors that influence in vitro fertilization success rates, such as ovarian reserve, documented by the local multidisciplinary fertility teams (39). Additionally, this meant that we could only report on cycles with oocyte retrieval, meaning that there are possibly some cycles without oocyte retrieval that we could not take into account in our analysis, thus overestimating the live birth rate. Most importantly, half of the couples proceeded with preimplantation genetic testing, which could have created another sample bias. Although the 65% rate of at least one live birth per couple reported in this study is hopeful for couples seeking preimplantation genetic testing for monogenic kidney disease, this number should be used in counseling with caution because of the small sample size and possible biases.
Thirty-eight percent of referred couples did not proceed with preimplantation genetic testing, which is a higher percentage than the previously reported 17% in a Belgian cohort of 65 patients with ADPKD (21). This difference is likely due to our earlier moment of inclusion, namely at their first referral for counseling in the MUMC+, because the number of couples who opted out is similar to the nationwide PGT The Netherlands data (35). We underestimate the overall decline rate because we could not collect information on patients who were counseled by their local geneticist or nephrologist and chose not to be referred for more extensive preimplantation genetic testing counseling in the MUMC+.
In our cohort, the main patient-reported reasons for declining preimplantation genetic testing were the time investment and perceived relatively low chance of a pregnancy going >12 weeks gestational age after preimplantation genetic testing, which is consistent with literature (21,30,40). Interestingly, in our cohort, time from the moment of first counseling to delivery of the first child was similar for preimplantation genetic testing couples and couples who did not have preimplantation genetic testing. The relatively healthy state of our cohort (88% in early CKD) could also have influenced decision making. The effect of index patient disease state is illustrated by a couple who did not choose preimplantation genetic testing or invasive prenatal diagnostics for their first pregnancy. Soon after having their first child, the index patient underwent a kidney transplant. This shifted their perspective on disease severity and burden in such a way that the couple subsequently decided that they did not want to pass on this disease to their further offspring and opted for preimplantation genetic testing for the next pregnancy. A case like this underscores the importance of periodic counseling on preimplantation genetic testing of patients in the reproductive age.
Counseling patients with monogenic kidney disease on preimplantation genetic testing, its waiting time, and success rates should also include information on the technical limitations to preimplantation genetic testing. For example, preimplantation genetic testing is only an option if the disease-causing mutation in the family can be identified and a single-cell genetic test can be developed (41). Additionally, in some ADPKD families, hypomorphic alleles or modifier variants complicate genetic counseling and preimplantation genetic testing because penetrance may be variable (42–44). Finally, prospective parents are counseled on the maternal health risks related to the in vitro fertilization/intra-cytoplastmatic sperm injection (ICSI) needed for preimplantation genetic testing (e.g., ovarian hyperstimulation syndrome and postretrieval bleeding or infection) and risks related to pregnancy in patients with CKD in general, which rise with advancement in CKD stage (14,30,45,46).
We recommend that all prospective parents from monogenic kidney disease families are counseled on reproductive options, including preimplantation genetic testing, as a part of standard care (47). If the couples express interest in preimplantation genetic testing or invasive prenatal diagnostics or ask for more in-depth reproductive counseling, they should be referred to a specialized genetic counseling unit. In a study among 96 patients with ADPKD in the United Kingdom, 63% of patients with kidney failure reported that they would have considered preimplantation genetic testing, and 18% reported that they would consider invasive prenatal diagnostics and termination of pregnancy (48). In addition, 68% of patients thought preimplantation genetic testing should be offered to patients with ADPKD, regardless of whether they would consider this option for themselves (48). The fact that the majority of affected parents in our cohort had CKD stage 1 underscores the notion that patients are interested in preimplantation genetic testing regardless of their disease stage (10).
Our recommendation is in line with the Kidney Disease Improving Global Outcomes consensus report on ADPKD stating that preimplantation genetic testing should be part of reproductive counseling of patients with ADPKD as “these decisions are for the patients and/or parents to make,” although access to this technology varies across countries (10,49). However, a study among clinicians revealed that 93% of clinical geneticists inform patients with ADPKD about the option of preimplantation genetic testing, whereas only 41% of nephrologists and 23% of pediatric nephrologists discuss preimplantation genetic testing (47). Increased awareness of preimplantation genetic testing for monogenic kidney disease among (pediatric) nephrologists is required (for example, through checklists for patients and guidelines for doctors that include discussing genetic testing and family planning) to standardize care for patients with monogenic kidney disease and families (49).
In conclusion, we provide the first extensive overview of preimplantation genetic testing referrals for monogenic kidney disease. Our analysis includes monogenic kidney disease indications, considerations of prospective parents, and the uptake and results of the procedure. Since 2009, there has been an increase in referrals for monogenic kidney disease in particular for adult-onset conditions. The percentage of unaffected live born children resulting from preimplantation genetic testing in monogenic kidney disease is high in our cohort, likely due to sample bias. Still, the uptake of 53% could indicate that decisions regarding preimplantation genetic testing are complex for prospective parents. Our data can aid in counseling prospective parents from families with monogenic kidney disease on the option of preimplantation genetic testing. This enables couples to make informed decisions in line with their personal, cultural, and moral backgrounds and beliefs.
Disclosures
All authors have nothing to disclose.
Funding
This work was funded by Nierstichting grant 14OP15 (to A.M. van Eerde).
Supplementary Material
Acknowledgments
Dr. Christine E.M. de Die-Smulders, Dr. Nine V.A.M. Knoers, Dr. A. Titia Lely, Rozemarijn Snoek MSc., and Dr. Albertien M. van Eerde provided the research idea and study design; Dr. Jos C.M.F. Dreesen, Dr. Aimée D.C. Paulussen, Ms. Cindy E. Simcox, Rozemarijn Snoek MSc, Franka van Reekum, and Dr. Theodora C. van Tilborg acquired data; Dr. Klaske D. Lichtenbelt, Rozemarijn Snoek MSc, Dr. Marijn F. Stokman, and Dr. Albertien M. van Eerde provided data analysis/interpretation; Rozemarijn Snoek MSc provided statistical analysis; Rozemarijn Snoek MSc, Dr. Marijn F. Stokman, and Dr. Albertien M. van Eerde drafted the manuscript; all authors revised and approved the manuscript; and all authors contributed important intellectual content during manuscript drafting or revision and accept accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03550320/-/DCSupplemental.
References
- 1.Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, Hobbs FD: Global prevalence of chronic kidney disease—A systematic review and meta-analysis. PLoS One 11: e0158765, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carrero J-J, Hecking M, Ulasi I, Sola L, Thomas B: Chronic kidney disease, gender, and access to care: A global perspective. Semin Nephrol 37: 296–308, 2017. [DOI] [PubMed] [Google Scholar]
- 3.Kuznik A, Mardekian J, Tarasenko L: Evaluation of cardiovascular disease burden and therapeutic goal attainment in US adults with chronic kidney disease: An analysis of national health and nutritional examination survey data, 2001-2010. BMC Nephrol 14: 132, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connaughton DM, Kennedy C, Shril S, Mann N, Murray SL, Williams PA, Conlon E, Nakayama M, van der Ven AT, Ityel H, Kause F, Kolvenbach CM, Dai R, Vivante A, Braun DA, Schneider R, Kitzler TM, Moloney B, Moran CP, Smyth JS, Kennedy A, Benson K, Stapleton C, Denton M, Magee C, O’Seaghdha CM, Plant WD, Griffin MD, Awan A, Sweeney C, Mane SM, Lifton RP, Griffin B, Leavey S, Casserly L, de Freitas DG, Holian J, Dorman A, Doyle B, Lavin PJ, Little MA, Conlon PJ, Hildebrandt F: Monogenic causes of chronic kidney disease in adults. Kidney Int 95: 914–928, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vivante A, Hildebrandt F: Exploring the genetic basis of early-onset chronic kidney disease. Nat Rev Nephrol 12: 133–146, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lata S, Marasa M, Li Y, Fasel DA, Groopman E, Jobanputra V, Rasouly H, Mitrotti A, Westland R, Verbitsky M, Nestor J, Slater LM, D’Agati V, Zaniew M, Materna-Kiryluk A, Lugani F, Caridi G, Rampoldi L, Mattoo A, Newton CA, Rao MK, Radhakrishnan J, Ahn W, Canetta PA, Bomback AS, Appel GB, Antignac C, Markowitz GS, Garcia CK, Kiryluk K, Sanna-Cherchi S, Gharavi AG: Whole-exome sequencing in adults with chronic kidney disease: A pilot study. Ann Intern Med 168: 100–109, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hildebrandt F: Genetic kidney diseases. Lancet 375: 1287–1295, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Büscher AK, Konrad M, Nagel M, Witzke O, Kribben A, Hoyer PF, Weber S: Mutations in podocyte genes are a rare cause of primary FSGS associated with ESRD in adult patients. Clin Nephrol 78: 47–53, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Stokman MF, Renkema KY, Giles RH, Schaefer F, Knoers NV, van Eerde AM: The expanding phenotypic spectra of kidney diseases: Insights from genetic studies. Nat Rev Nephrol 12: 472–483, 2016. [DOI] [PubMed] [Google Scholar]
- 10.van Eerde AM, Krediet CTP, Rookmaaker MB, van Reekum FE, Knoers NV, Lely AT: Pre-pregnancy advice in chronic kidney disease: Do not forget genetic counseling. Kidney Int 90: 905–906, 2016. [DOI] [PubMed] [Google Scholar]
- 11.Groopman EE, Rasouly HM, Gharavi AG: Genomic medicine for kidney disease. Nat Rev Nephrol 14: 83–104, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross O, Licht C, Anders HJ, Hoppe B, Beck B, Tönshoff B, Höcker B, Wygoda S, Ehrich JH, Pape L, Konrad M, Rascher W, Dötsch J, Müller-Wiefel DE, Hoyer P, Knebelmann B, Pirson Y, Grunfeld JP, Niaudet P, Cochat P, Heidet L, Lebbah S, Torra R, Friede T, Lange K, Müller GA, Weber M; Study Group Members of the Gesellschaft für Pädiatrische Nephrologie : Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int 81: 494–501, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Münch J, Grohmann M, Lindner TH, Bergmann C, Halbritter J: Diagnosing FSGS without kidney biopsy - A novel INF2-mutation in a family with ESRD of unknown origin. BMC Med Genet 17: 73, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snoek R, van der Graaf R, Meinderts JR, van Reekum F, Bloemenkamp KWM, Knoers NVAM, van Eerde AM, Lely AT: Pregnancy in advanced kidney disease: Clinical practice considerations on a challenging combination. Nephron 144: 185–189, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alfirevic Z, Navaratnam K, Mujezinovic F: Amniocentesis and chorionic villus sampling for prenatal diagnosis. Cochrane Database Syst Rev 9: CD003252, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harton GL, De Rycke M, Fiorentino F, Moutou C, SenGupta S, Traeger-Synodinos J, Harper JC; European Society for Human Reproduction and Embryology (ESHRE) PGD Consortium : ESHRE PGD consortium best practice guidelines for amplification-based PGD. Hum Reprod 26: 33–40, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Rechitsky S, Verlinsky O, Kuliev A: PGD for cystic fibrosis patients and couples at risk of an additional genetic disorder combined with 24-chromosome aneuploidy testing. Reprod Biomed Online 26: 420–430, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Kuliev A, Pomerantseva E, Polling D, Verlinsky O, Rechitsky S: PGD for inherited cardiac diseases. Reprod Biomed Online 24: 443–453, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Rechitsky S, Verlinsky O, Chistokhina A, Sharapova T, Ozen S, Masciangelo C, Kuliev A, Verlinsky Y: Preimplantation genetic diagnosis for cancer predisposition. Reprod Biomed Online 5: 148–155, 2002. [DOI] [PubMed] [Google Scholar]
- 20.De Rycke M, Georgiou I, Sermon K, Lissens W, Henderix P, Joris H, Platteau P, Van Steirteghem A, Liebaers I: PGD for autosomal dominant polycystic kidney disease type 1. Mol Hum Reprod 11: 65–71, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Berckmoes V, Verdyck P, De Becker P, De Vos A, Verheyen G, Van der Niepen P, Verpoest W, Liebaers I, Bonduelle M, Keymolen K, De Rycke M: Factors influencing the clinical outcome of preimplantation genetic testing for polycystic kidney disease. Hum Reprod 34: 949–958, 2019. [DOI] [PubMed] [Google Scholar]
- 22.Li W, Ma Y, Yu S, Sun N, Wang L, Chen D, Yang G, Lu S, Li Y, Yang B, Mei C: The mutation-free embryo for in vitro fertilization selected by MALBAC-PGD resulted in a healthy live birth from a family carrying PKD 1 mutation. J Assist Reprod Genet 34: 1653–1658, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renbaum P, Brooks B, Kaplan Y, Eldar-Geva T, Margalioth EJ, Levy-Lahad E, Altarescu G: Advantages of multiple markers and polar body analysis in preimplantation genetic diagnosis for Alagille disease. Prenat Diagn 27: 317–321, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Altarescu G, Eldar Geva T, Brooks B, Margalioth E, Levy-Lahad E, Renbaum P: PGD on a recombinant allele: Crossover between the TSC2 gene and ‘linked’ markers impairs accurate diagnosis. Prenat Diagn 28: 929–933, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Vanneste E, Melotte C, Debrock S, D’Hooghe T, Brems H, Fryns JP, Legius E, Vermeesch JR: Preimplantation genetic diagnosis using fluorescent in situ hybridization for cancer predisposition syndromes caused by microdeletions. Hum Reprod 24: 1522–1528, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Obradors A, Fernández E, Rius M, Oliver-Bonet M, Martínez-Fresno M, Benet J, Navarro J: Outcome of twin babies free of Von Hippel-Lindau disease after a double-factor preimplantation genetic diagnosis: Monogenetic mutation analysis and comprehensive aneuploidy screening. Fertil Steril 91: 933.e1-933.e7, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Lau EC, Janson MM, Roesler MR, Avner ED, Strawn EY, Bick DP: Birth of a healthy infant following preimplantation PKHD1 haplotyping for autosomal recessive polycystic kidney disease using multiple displacement amplification. J Assist Reprod Genet 27: 397–407, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogur G, Zenker M, Tosun M, Ekici F, Schanze D, Ozyilmaz B, Malatyalioglu E: Clinical and molecular studies in two families with Fraser syndrome: A new FRAS1 gene mutation, prenatal ultrasound findings and implications for genetic counselling. Genet Couns 22: 233–244, 2011. [PubMed] [Google Scholar]
- 29.Lu Y, Peng H, Jin Z, Cheng J, Wang S, Ma M, Lu Y, Han D, Yao Y, Li Y, Yuan H: Preimplantation genetic diagnosis for a Chinese family with autosomal recessive Meckel-Gruber syndrome type 3 (MKS3). PLoS One 8: e73245, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy EL, Droher ML, DiMaio MS, Dahl NK: Preimplantation genetic diagnosis counseling in autosomal dominant polycystic kidney disease. Am J Kidney Dis 72: 866–872, 2018. [DOI] [PubMed] [Google Scholar]
- 31.National Kidney Foundation : 2015 Update. Am J Kidney Dis 66: 884–930, 2015. [DOI] [PubMed] [Google Scholar]
- 32.Altarescu G, Beeri R, Eiges R, Epsztejn-Litman S, Eldar-Geva T, Elstein D, Zimran A, Margalioth EJ, Levy-Lahad E, Renbaum P: Prevention of lysosomal storage diseases and derivation of mutant stem cell lines by preimplantation genetic diagnosis. Mol Biol Int 2012: 797342, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gigarel N, Frydman N, Burlet P, Kerbrat V, Tachdjian G, Fanchin R, Antignac C, Frydman R, Munnich A, Steffann J: Preimplantation genetic diagnosis for autosomal recessive polycystic kidney disease [published correction appears in Reprod Biomed Online 16: 463, 2008]. Reprod Biomed Online 16: 152–158, 2008 [DOI] [PubMed] [Google Scholar]
- 34.De Rycke M, Goossens V, Kokkali G, Meijer-Hoogeveen M, Coonen E, Moutou C: ESHRE PGD consortium data collection XIV-XV: Cycles from January 2011 to December 2012 with pregnancy follow-up to October 2013. Hum Reprod 32: 1974–1994, 2017. [DOI] [PubMed] [Google Scholar]
- 35.Nederland PGD: Annual Report PGD Nederland 2017, Maastricht, Limburg, The Netherlands, PGD Nederland, 2017 [Google Scholar]
- 36.Genoff Garzon MC, Rubin LR, Lobel M, Stelling J, Pastore LM: Review of patient decision-making factors and attitudes regarding preimplantation genetic diagnosis. Clin Genet 94: 22–42, 2018. [DOI] [PubMed] [Google Scholar]
- 37.Valdrez K, Silva S, Coelho T, Alves E: Awareness and motives for use and non-use of preimplantation genetic diagnosis in familial amyloid polyneuropathy mutation carriers. Prenat Diagn 34: 886–892, 2014. [DOI] [PubMed] [Google Scholar]
- 38.Quinn G, Vadaparampil S, Wilson C, King L, Choi J, Miree C, Friedman S: Attitudes of high-risk women toward preimplantation genetic diagnosis. Fertil Steril 91: 2361–2368, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Leijdekkers JA, Eijkemans MJC, van Tilborg TC, Oudshoorn SC, McLernon DJ, Bhattacharya S, Mol BWJ, Broekmans FJM, Torrance HL; OPTIMIST group : Predicting the cumulative chance of live birth over multiple complete cycles of in vitro fertilization: An external validation study. Hum Reprod 33: 1684–1695, 2018. [DOI] [PubMed] [Google Scholar]
- 40.Gebhart MB, Hines RS, Penman A, Holland AC: How do patient perceived determinants influence the decision-making process to accept or decline preimplantation genetic screening? Fertil Steril 105: 188–193, 2016. [DOI] [PubMed] [Google Scholar]
- 41.Harris PC, Torres VE: In: GeneReviews, edited by Pagon RA, Adam MP, Ardinger HH, Wallace SE, Bean LJH, Stephens K, et al., Seattle, WA, University of Washington, 1993 [Google Scholar]
- 42.Pei Y, Lan Z, Wang K, Garcia-Gonzalez M, He N, Dicks E, Parfrey P, Germino G, Watnick T: A missense mutation in PKD1 attenuates the severity of renal disease. Kidney Int 81: 412–417, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergmann C, von Bothmer J, Ortiz Brüchle N, Venghaus A, Frank V, Fehrenbach H, Hampel T, Pape L, Buske A, Jonsson J, Sarioglu N, Santos A, Ferreira JC, Becker JU, Cremer R, Hoefele J, Benz MR, Weber LT, Buettner R, Zerres K: Mutations in multiple PKD genes may explain early and severe polycystic kidney disease. J Am Soc Nephrol 22: 2047–2056, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossetti S, Consugar MB, Chapman AB, Torres VE, Guay-Woodford LM, Grantham JJ, Bennett WM, Meyers CM, Walker DL, Bae K, Zhang QJ, Thompson PA, Miller JP, Harris PC; CRISP Consortium : Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 18: 2143–2160, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Wiles KS, Nelson-Piercy C, Bramham K: Reproductive health and pregnancy in women with chronic kidney disease. Nat Rev Nephrol 14: 165–184, 2018. [DOI] [PubMed] [Google Scholar]
- 46.Piccoli GB, Cabiddu G, Attini R, Vigotti FN, Maxia S, Lepori N, Tuveri M, Massidda M, Marchi C, Mura S, Coscia A, Biolcati M, Gaglioti P, Nichelatti M, Pibiri L, Chessa G, Pani A, Todros T: Risk of adverse pregnancy outcomes in women with CKD. J Am Soc Nephrol 26: 2011–2022, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Rechter S, Kringen J, Janssens P, Liebau MC, Devriendt K, Levtchenko E, Bergmann C, Jouret F, Bammens B, Borry P, Schaefer F, Mekahli D: Clinicians’ attitude towards family planning and timing of diagnosis in autosomal dominant polycystic kidney disease. PLoS One 12: e0185779, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swift O, Vilar E, Rahman B, Side L, Gale DP: Attitudes in patients with autosomal dominant polycystic kidney disease toward prenatal diagnosis and preimplantation genetic diagnosis. Genet Test Mol Biomarkers 20: 741–746, 2016. [DOI] [PubMed] [Google Scholar]
- 49.Chapman AB, Devuyst O, Eckardt K-U, Gansevoort RT, Harris T, Horie S, Kasiske BL, Odland D, Pei Y, Perrone RD, Pirson Y, Schrier RW, Torra R, Torres VE, Watnick T, Wheeler DC; Conference Participants : Autosomal-dominant polycystic kidney disease (ADPKD): Executive summary from a kidney disease: Improving global outcomes (KDIGO) controversies conference. Kidney Int 88: 17–27, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.