Figure 1.
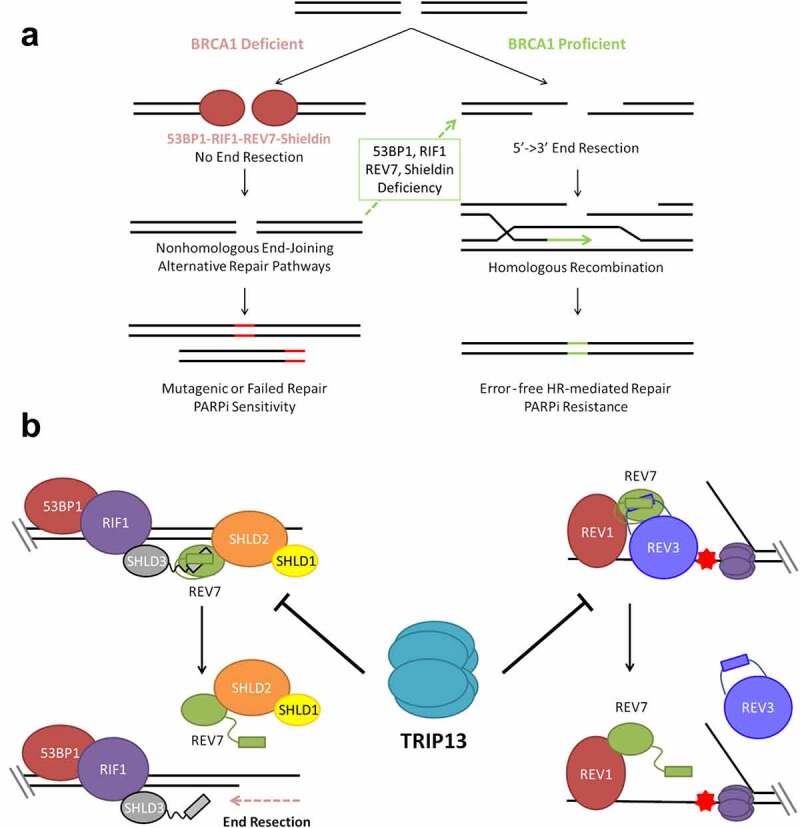
TRIP13 promotes PARP inhibitor sensitivity by disassembling REV7-Shieldin. (a) BRCA1 promotes 5ʹ-3ʹ DNA end resection, the critical first step in double strand break (DSB) repair through homologous recombination. In the absence of BRCA1; 53BP1, RIF1, REV7 and Shieldin inhibit DNA end resection, preventing repair by HR. End resection and HR can be restored in BRCA1-deficient cells by concurrent deficiency of any component of this anti-resection axis. Without HR repair, cells are unable to properly repair all DSBs, particularly those that arise during S phase leading to hypersensitivity to PARP inhibition and other DNA damaging therapies. (b) The Shieldin complex assembles at the break site through sequential recruitment of 53BP1, RIF1, SHLD3, REV7 and SHLD1-SHLD2 (left). REV7 is also recruited to sites of replication blockages, where it interacts with REV1 and REV3 as part of the DNA Polymerase ζ complex (right). TRIP13 catalyzes an inactivating conformational change of REV7, thereby inhibiting both the Shieldin complex and the Polymerase ζ complex.
