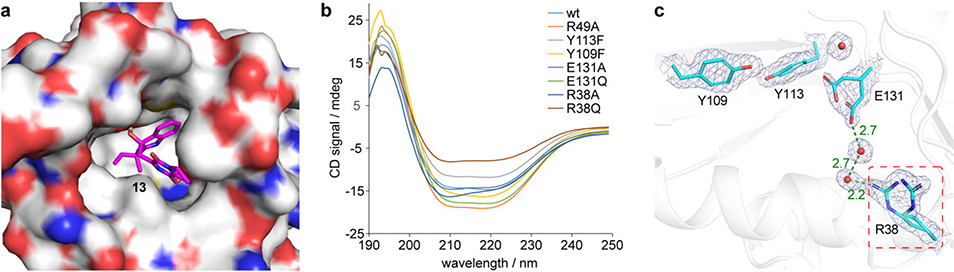
Extended Data Figure 6. Docking results and CD spectra of BvnE.
a, Docking complex of BvnE with compound 13 (magenta). b, CD spectra of purified wild-type and mutant BvnE proteins. c, Electron density (2Fo-Fc, 1σ) for the active site residues of BvnE (PDB ID code: 6U9I) shows conformational flexibility at Arg38 and Glu131 suggestive of potential active site remodeling during catalysis. Arg38 (red box) which is essential for catalytic activity does not make direct interactions with the docked ligand or active site residues. However, the structure reveals two ordered water molecules (red spheres) in a hydrogen bonding network between Arg38 and Glu131. These binding poses reveal possible key interactions between the ligand and BvnE.