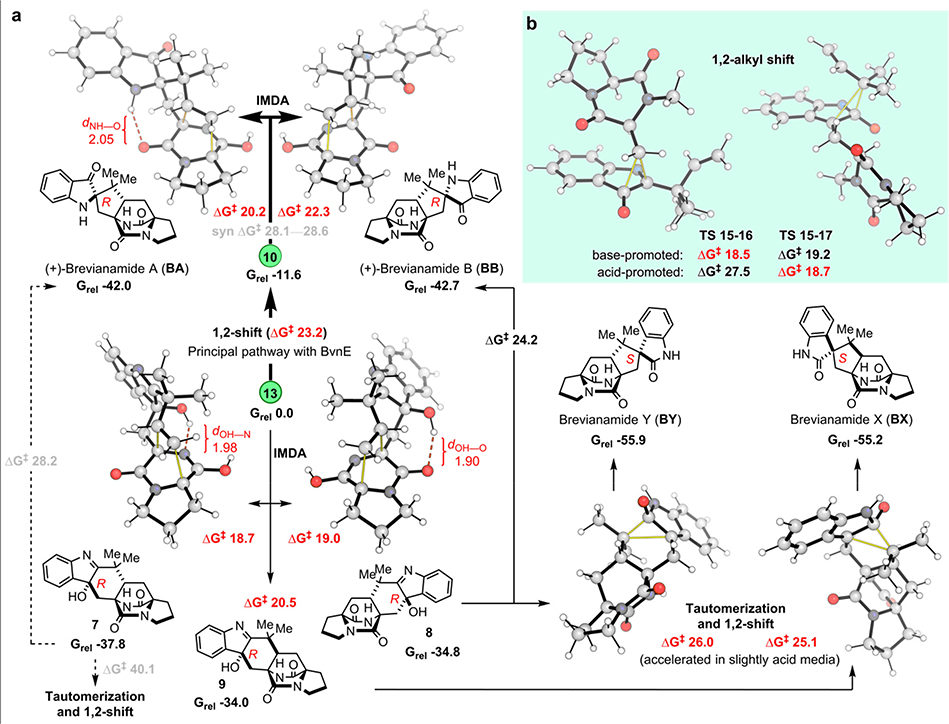
Fig. 6. Quantum chemical calculation results.
a, anti-Selective IMDA transition structures and products accessible from intermediates 13 and 10. b, 1,2-alkyl shift (semi-pinacol) transition structures for the migration of CH2-dioxopiperazine (LHS) and reverse-prenyl (RHS) groups. Computed Gibbs energies are in kcal/mol and highlighted distances in Å. Normal lines follow the major pathways obtained under Pb-bvnE-KO conditions. Pathways in bold correspond to the major reactivity obtained when using BvnE (switch in reactivity). Dashed lines represent pathways with prohibitively high activation energies under the reaction conditions.