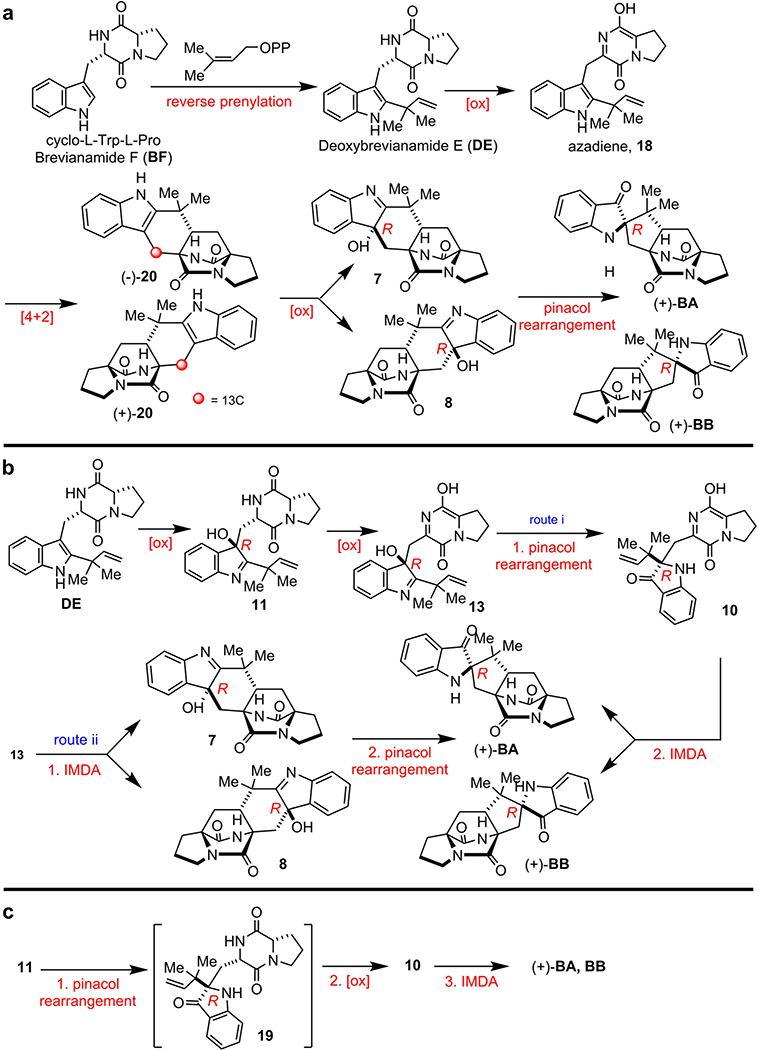
Extended Data Figure 3. Biogenetic proposals for Brevianamide A (BA) and Brevianamide B (BB).
a, Early biosynthetic proposal suggested and interrogated by Williams et. al.13 b, Original biogenesis proposed by Porter and Sammes (via 7).14 c, More recent biosynthetic proposals suggested by Williams et al..5,6,10 Several biogenetic hypotheses based on the pioneering proposal first suggested by Porter and Sammes in 197014 reasoned that the bicyclo[2.2.2]diazaoctane core arises via an intramolecular [4+2] hetero-Diels-Alder (IMDA) construction.5–10,36 We experimentally interrogated the biogenetic proposal a, through the synthesis of 13C-labeled putative Diels-Alder products (±)-20, but could not detect incorporation into either BA or BB in cultures of Pb. Based on these results, we then suggested the biosynthetic pathways illustrated in b and c. The fundamental difference between the biogenesis described in a, and that in b and c, is the sequential timing of the indole oxidation, the semi-pinacol rearrangement and the crucial IMDA reactions. Thus, it remained conceivable that oxidation of DE to the (R)-hydroxyindolenine provides species 11, which can suffer several fates. One is N-C ring closure to co-metabolite Brevianamide E (BE); a second possibility shown in b is oxidation and tautomerization to the azadiene species 13, which can suffer IMDA cyclization providing 7 and 8, then undergo a final semi-pinacol rearrangement to furnish BA and BB (route i). Alternatively, azadiene 13 could first suffer semi-pinacol rearrangement to 10, which then undergoes the IMDA construction to generate BA and BB (route ii). Another possibility c involves (R)-hydroxyindolenine 11, which proceeds through a semi-pinacol rearrangement to the indoxyl species 19, followed by further oxidation to the azadiene species 10 and subsequent IMDA to furnish BA and BB. Experimental supports to distinguish between these proposed pathways, all of which embrace the relative and absolute stereochemistry of BA and BB, have remained unresolved until this work.