Abstract
Objectives
The aim of this study was to assess the co-seasonality and co-detection of respiratory viral infections and bacteraemia in children since the introduction of the 13-valent pneumococcal conjugate vaccine (PCV13).
Methods
Children <18 years old were eligible for inclusion if they had a respiratory infection and a positive PCR-based assay for respiratory viruses as well as a positive blood culture between 2010 and 2018 at a single referral centre in the United States, regardless of their underlying medical condition or antibiotic treatment history. Monthly incidence rates of respiratory viruses and bacteraemia were analysed with a seasonal-trend decomposition procedure based on loess (STL) and cross-correlation functions using time series regression modelling.
Results
We identified 7415 unique positive respiratory virus tests, including 2278 respiratory syncytial virus (RSV) (31%), 1825 influenza viruses (24%), 1036 parainfluenza viruses (14%), 1017 human metapneumovirus (hMPV) (14%), 677 seasonal coronaviruses (9%), and 582 adenoviruses (8%), together with a total of 11 827 episodes of bacteraemia. Significant co-seasonality was found between all-cause bacteraemia and RSV (OR = 1.76, 95%CI 1.50–2.06, p < 0.001), influenza viruses (OR = 1.38, 95%CI 1.13–1.68, p 0.002), and seasonal coronaviruses (OR = 1.18, 95%CI 1.09–1.28, p < 0.001), respectively. Analysis of linked viral–bacterial infections in individual children indicated that the rate ratio (RR) of bacteraemia associated with hMPV (RR = 2.73, 95%CI 1.12–6.85, p 0.019) and influenza (RR = 2.61, 95%CI 1.21–6.11, p 0.013) were more than double that of RSV. Staphylococcus aureus and Streptococcus pneumoniae were the most commonly identified pathogens causing bacteraemia.
Conclusions
There is a significant association between hMPV and influenza viruses and bacteraemia of all causes in hospitalized children at a single paediatric centre in the United States. Large multicentre studies are needed to confirm these findings and to elucidate the mechanisms by which hMPV potentiates the virulence and invasive capacity of diverse bacteria.
Keywords: Bacteraemia, Child, Co-detection, Human metapneumovirus, Influenza, RSV, Seasonality
Introduction
Children with respiratory viral infections are susceptible to infection with bacteria that may cause pyogenic complications such as empyema, necrotizing pneumonia and bacteraemia [1]. Since the influenza pandemic of 1918, Streptococcus pneumoniae and Staphylococcus aureus have been recognized as the predominant causes of invasive bacterial infections complicating influenza infections [2].
The direct relationship between respiratory viruses and bacteraemia in children remains poorly defined, especially since the introduction of the seven-valent pneumococcal conjugate vaccine (PCV7) in the United States in 2000 [3,4]. A study conducted in children before the implementation of PCV13 demonstrated significant associations between invasive pneumococcal disease (IPD) and influenza viruses and respiratory syncytial virus (RSV), as well as human metapneumovirus (hMPV), which was a novel observation [5].
The deployment of PCV13 in the United States in 2010 has led to a substantial decline in IPD, but it is not currently known which bacteria complicate respiratory viral infections [3]. We hypothesized that respiratory viruses detected in hospitalized children are associated with multiple causes of bacteraemia. To test this hypothesis, we analysed the relationship between 16 respiratory viruses and all-cause bacteraemia in children at a single paediatric centre in the United States over the 8 years since the introduction of PCV13.
Methods
Research setting
This ecological study was conducted at Hasbro Children's Hospital in Providence, Rhode Island, which serves southern New England in the United States.
Study population
Children <18 years of age presenting with respiratory infections were enrolled if they underwent testing for respiratory viruses and bacteraemia in the emergency department, paediatric primary care clinic or during hospitalization from June 2010 to May 2018. Blood cultures and respiratory viral PCR assays were obtained at the discretion of attending physicians according to usual local practice for children with fever and respiratory symptoms. No systematic changes were made during the study period. Based on the knowledge that respiratory viruses incubate for up to 1 week and are shed for 14 days or longer [6,7], we made an a priori assumption that detection of a virus 2 weeks before or up to 1 week after a positive blood culture could potentially be causally associated with bacteraemia. Children were eligible regardless of their underlying medical condition or antibiotic treatment history. Aggregated laboratory results, season, and patients' ages were collated using TheraDoc (Premier, Charlotte, North Carolina, USA), an infection control software system.
Laboratory tests
PCR-based viral assays included the xTAG Respiratory Viral Panel (Luminex Corp., Austin, Texas, USA) from 2010 to 2017 and the ePlex Respiratory Pathogen Panel (GenMark Diagnostics, Carlsbad, California, USA) from 2017 to 2018, both of which test for 17 viruses. Considering that these assays cannot distinguish between rhinoviruses and enteroviruses, we decided in advance to exclude them from the analyses. The following bacteria were considered non-pathogenic unless they were isolated from two or more blood cultures: coagulase-negative staphylococci, Corynebacterium spp., Bacillus spp., and Cutibacterium acnes. Only the first episode of infection was included if a pathogen was reported on more than one occasion within a 4-week period. Information about pneumococcal serotypes was not available because serotyping is not routinely performed in Rhode Island.
Statistical analyses
We constructed a longitudinal database to track monthly incidence of respiratory viruses and bacteraemia. A filtering procedure—called a seasonal-trend decomposition procedure based on loess (STL)—was conducted separately for respiratory viruses and bacteria in order to decompose and smooth time series data with seasonal, trend and remaining components [8]. Cross-correlation functions were applied using time series regression modelling to determine the highest correlation between overall incidence of various respiratory viruses and bacteraemia. We calculated the incidence of cases with viral–bacterial co-detections as well as a rate ratio (RR) of various respiratory viruses relative to that of RSV. RR was calculated using the median unbiased estimator method. Statistical analyses were performed using R (version 3.4.3; R Development Core Team, Vienna, Austria).
Ethics statement
The Institutional Review Board at Rhode Island Hospital provided ethics approval for this study and exemption from informed consent.
Results
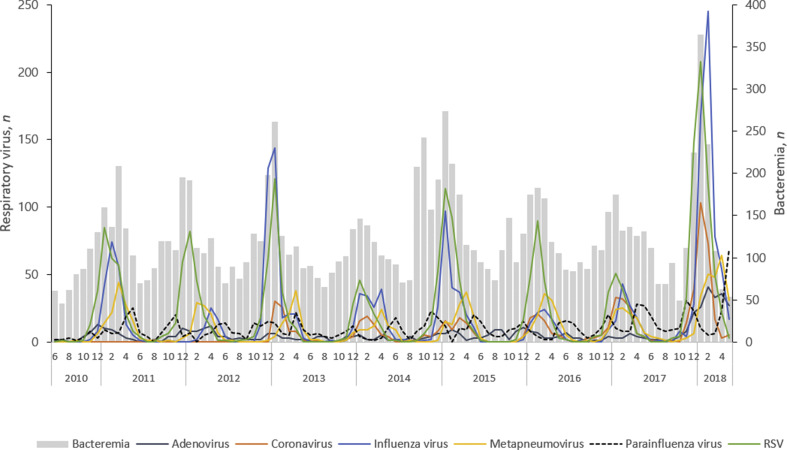
During an 8-year period from 2010, we identified 7415 unique positive respiratory virus tests, including 2278 RSV (31%), 1825 influenza viruses (24%), 1036 parainfluenza viruses (14%), 1017 hMPV (14%), 677 seasonal coronaviruses (9%), and 582 adenoviruses (8%) (Fig. 1 ). During the same period, a total of 11 827 episodes of bacteraemia from the entire hospital were identified. Seasonal coronaviruses, influenza viruses and RSV peaked in the winter, which coincided with the peak incidence of all-cause bacteraemia. The highest incidence of hMPV and spring-onset parainfluenza viruses lagged that of other viruses by 4–8 weeks, and typically occurred annually in April or May.
Fig. 1.
Co-seasonality of respiratory viruses (line graph) and bacteraemia (bar graph), 2010–2018.
The cross correlations between respiratory viruses and all-cause bacteraemia revealed the highest effect sizes for RSV (OR = 1.76, p < 0.001), influenza viruses (OR = 1.38, p 0.002), and seasonal coronaviruses (OR = 1.18, p < 0.001) (Table 1 ). There were no significant seasonal associations between adenovirus, hMPV, or parainfluenza viruses and bacteraemia.
Table 1.
Co-seasonality and co-detection of respiratory viruses and bacteraemia in children, 2010–2018
| Respiratory viruses | Viral URT detection and bacteraemia co-seasonality |
Viral URT detection and bacteraemia |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | Adjusted R2 | p | n/N | Incidence % | RR (vs. RSV) | 95%CI | p (versus RSV) | |
| Adenovirus | 1.02 | 0.99–1.06 | 0.320 | 0.216 | 2/582 | 0.34 | 0.92 | 0.13–3.65 | 0.858 |
| Coronaviruses | 1.18 | 1.09–1.28 | 0.651 | <0.001 | 5/677 | 0.74 | 1.90 | 0.57–5.58 | 0.254 |
| Influenza viruses | 1.38 | 1.13–1.68 | 0.591 | 0.002 | 19/1825 | 1.04 | 2.61 | 1.21–6.11 | 0.013 |
| hMPV | 0.99 | 0.92–1.05 | 0.658 | 0.669 | 11/1017 | 1.08 | 2.73 | 1.12–6.85 | 0.019 |
| Parainfluenza viruses | 1.05 | 0.99–1.13 | 0.120 | 0.117 | 9/1036 | 0.87 | 2.20 | 0.85 – 5.71 | 0.086 |
| RSV | 1.76 | 1.50–2.06 | 0.797 | <0.001 | 9/2278 | 0.40 | 1 | — | — |
Bold figures denote significant values. CI, confidence interval; hMPV, human metapneumovirus; OR, odds ratio; RR, rate ratio; RSV, respiratory syncytial virus; URT, upper respiratory tract.
We used RSV as a reference for computing RR because it had a low proportion of bacteraemia episodes. Children with hMPV had the highest proportion of bacterial co-detections with an RR of 2.7 relative to RSV (p 0.019). Similarly, the RR for bacteraemia associated with influenza viruses was 2.6 compared with RSV (p 0.013). Adenoviruses, seasonal coronaviruses and parainfluenza viruses had respective RRs of 0.92, 1.90 and 2.20 relative to RSV, but the differences were not statistically significant (Table 1).
S. aureus (n = 15) and S. pneumoniae (n = 12) were the mostly commonly identified pathogens causing bacteraemia (Supplementary Material Table S1).
Discussion
We observed that the seasonality of coronaviruses, influenza viruses and RSV strongly correlated with that of bacteraemia among hospitalized children. These findings corroborate those of other investigators [5,9] and indicate that the co-seasonality of respiratory viruses and bacteria is conducive to concurrent host colonization. After we linked episodes of viral and bacterial infections in individual children, we found that the proportion of co-detections was generally low, ranging from 0.4% for RSV to 1.1% for hMPV, which is within the range found in other recent studies (0.4–1.6%) [[10], [11], [12], [13], [14]]. However, children with hMPV or influenza viruses had more than double the rate of bacteraemia compared with RSV.
This report validates the association between influenza and hMPV and bacteraemia that was reported by Ampofo et al. [5] before the introduction of PCV13. In addition to S. pneumoniae, we identified S. aureus and a variety of other bacteria, which emphasizes the importance of emerging non-vaccine-preventable pathogens. In addition to influenza, hMPV is known to cause degenerative changes in the lower respiratory epithelium, potentially permitting colonizing bacteria to invade; it also impairs signalling at the immunological synapse between dendritic cells and T cells, potentially disrupting host defences, and this may explain its virulence [4,15].
This study is limited by its retrospective design and lack of detailed patient-level clinical data, such as evidence of upper versus lower respiratory tract infection, underlying comorbidities, antibiotic treatment history, and evidence of prior immunizations. Also, the role of multiple viruses detected simultaneously and the possible role of presumed contaminants was not assessed due to the aggregated nature of our data. Furthermore, the small number of patients with co-detections derived from a single institution limits the generalizability of these findings.
On the other hand, this is the first study to appraise the association between respiratory viruses and bacteraemia in children since the deployment of PCV13. Despite the small sample size, we employed a rigorous statistical approach to account for seasonal and secular trends, and our findings are consistent with those of a previous larger study conducted before the introduction of PCV13 [5].
In conclusion, we found a strong association between both hMPV and influenza and bacteraemia in children. Large multicentre studies are needed to confirm these findings and to elucidate the mechanisms by which hMPV potentiates the virulence and invasive capacity of diverse bacteria. Empirical antibacterial treatment of severely ill children infected with these viruses appears to be warranted.
Author contributions
YJC and ICM developed the study concept and design. All authors had full access to the data and take responsibility for the integrity of the data and accuracy of the data analysis. YJC was responsible for data collection. YJC and SP performed the statistical analyses. All authors assisted with data interpretation. YJC and ICM performed the literature search. YJC wrote the first draft of the manuscript. All authors have critically read and commented on draft versions of the report, and approved the final version.
Transparency declaration
The authors declare no competing interests. This work was supported by a grant from the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant number R25AI140490 to ICM).
Editor: J. Bielicki
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.09.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Michelow I.C., Olsen K., Lozano J., Rollins N.K., Duffy L.B., Ziegler T. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics. 2004;113:701–707. doi: 10.1542/peds.113.4.701. [DOI] [PubMed] [Google Scholar]
- 2.Morens D.M., Taubenberger J.K. The mother of all pandemics is 100 years old (and going strong)! Am J Public Health. 2018;108:1449–1454. doi: 10.2105/AJPH.2018.304631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore M.R., Link-Gelles R., Schaffner W., Lynfield R., Lexau C., Bennett N.M. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15:301–309. doi: 10.1016/S1473-3099(14)71081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang J.W., Lam T.T., Zaraket H., Lipkin W.I., Drews S.J., Hatchette T.F. Global epidemiology of non-influenza RNA respiratory viruses: data gaps and a growing need for surveillance. Lancet Infect Dis. 2017;17:e320–e326. doi: 10.1016/S1473-3099(17)30238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ampofo K., Bender J., Sheng X., Korgenski K., Daly J., Pavia A.T. Seasonal invasive pneumococcal disease in children: role of preceding respiratory viral infection. Pediatrics. 2008;122:229–237. doi: 10.1542/peds.2007-3192. [DOI] [PubMed] [Google Scholar]
- 6.Frank A.L., Taber L.H., Wells C.R., Wells J.M., Glezen W.P., Paredes A. Patterns of shedding of myxoviruses and paramyxoviruses in children. J Infect Dis. 1981;144:433–441. doi: 10.1093/infdis/144.5.433. [DOI] [PubMed] [Google Scholar]
- 7.Lessler J., Reich N.G., Brookmeyer R., Perl T.M., Nelson K.E., Cummings D.A. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect Dis. 2009;9:291–300. doi: 10.1016/S1473-3099(09)70069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleveland R., Cleveland W.J., Terpenning I. STL: a seasonal-trend decomposition procedure based on loess. J Off Stat. 1990;6:3–63. [Google Scholar]
- 9.Fisman D.N. Seasonality of infectious diseases. Annu Rev Public Health. 2007;28:127–143. doi: 10.1146/annurev.publhealth.28.021406.144128. [DOI] [PubMed] [Google Scholar]
- 10.Krief W.I., Levine D.A., Platt S.L., Macias C.G., Dayan P.S., Zorc J.J. Influenza virus infection and the risk of serious bacterial infections in young febrile infants. Pediatrics. 2009;124:30–39. doi: 10.1542/peds.2008-2915. [DOI] [PubMed] [Google Scholar]
- 11.Leung C.H., Tseng H.K., Wang W.S., Chiang H.T., Wu A.Y., Liu C.P. Clinical characteristics of children and adults hospitalized for influenza virus infection. J Microbiol Immunol Infect. 2014;47:518–525. doi: 10.1016/j.jmii.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Titus M.O., Wright S.W. Prevalence of serious bacterial infections in febrile infants with respiratory syncytial virus infection. Pediatrics. 2003;112:282–284. doi: 10.1542/peds.112.2.282. [DOI] [PubMed] [Google Scholar]
- 13.Bloomfield P., Dalton D., Karleka A., Kesson A., Duncan G., Isaacs D. Bacteraemia and antibiotic use in respiratory syncytial virus infections. Arch Dis Child. 2004;89:363–367. doi: 10.1136/adc.2003.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebel A., Ashkenazi-Hoffnung L., Shkalim V., Ben-Zvi H., Dotan M., Bilavsky E. Secondary bacteremia following adenovirus infection. Infect Dis (Lond) 2016;48:403–405. doi: 10.3109/23744235.2015.1122226. [DOI] [PubMed] [Google Scholar]
- 15.Cespedes P.F., Palavecino C.E., Kalergis A.M., Bueno S.M. Modulation of host immunity by the human metapneumovirus. Clin Microbiol Rev. 2016;29:795–818. doi: 10.1128/CMR.00081-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.