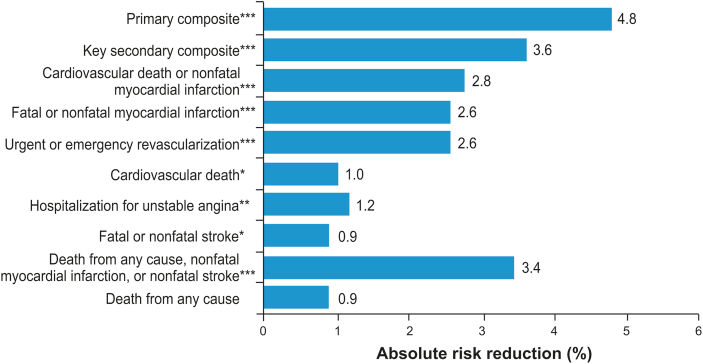
Fig. 2.
Absolute Risk Reduction (ARR) in REDUCE-IT Endpoints. Percentages were calculated by taking absolute differences in endpoint rates between icosapent ethyl and control groups [ARR = (n/N)placebo – (n/N)icosapent ethyl]. Primary composite endpoint events: cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina. Key secondary composite endpoint events: cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke [14]. ∗P < 0.05; ∗∗P ≤ 0.01; ∗∗∗P < 0.001 [14].