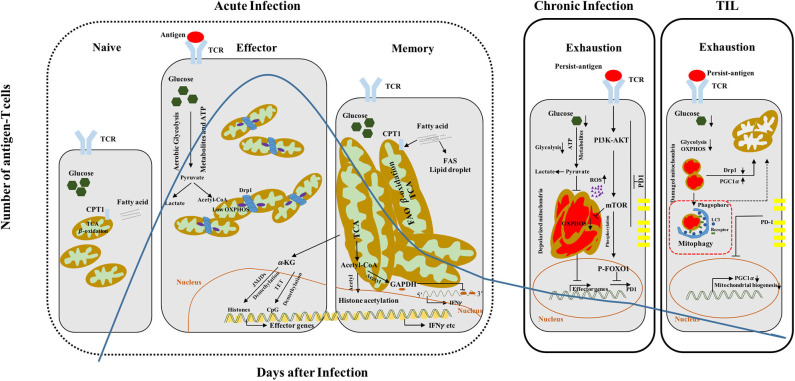
Figure 1.
CD8+ T cell differentiation and functionality couples with dynamic metabolic programming. Quiescent naive T cells display small and fragmented mitochondria, which utilize OXPHOS and FAO (CPT1, the rate-limiting enzyme) to maintain their survival. Although effector T cells switch from OXPHOS to aerobic glycolysis to support clonal expansion and effector functions, they also show transiently increased mitochondrial mass accompanied with Drp1-mediated mitochondrial fission. In contrast, memory T cells harbor more fused and elongated mitochondria, which have high spare respiratory capacity (SRC), and predominantly utilize fatty acid to produce energy via β-oxidation, accompanied with fatty acid synthesis (FAS). The effector/memory differentiation process can also be regulated by epigenetic modifications including mitochondrial metabolites (acetyl-CoA and α-KG). Acetyl-CoA provide donor acetyl to histone and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), which can be hyperacetylated respectively and promote interferon gamma (IFNγ) expression. α-KG acts as a cofactor of Jumonji C-domain-containing histone demethylases (JMJDs)/ten–eleven translocation (TET), and demethylates histone and DNA with the result of effector genes expression. Exhausted CD8+ T cells show multiple functional or structural alterations in mitochondria. Within CD8+ T cells during chronic infection, larger, structurally defective and depolarized mitochondria are accumulated and accompanied with high ROS production, leading to defective OXPHOS and loss of effector functions. TILs exhibit decreased mitochondrial mass but increased fragmented mitochondria with dysregulated structure and accumulation of ROS, resulting in defective glycolysis and OXPHOS. PD1 can negatively regulate the PGC1α and then inhibit mitochondrial biogenesis. Overexpression of PGC1α or knockdown Drp1 can recover the mitochondrial defects and functions and promote T cell effector functions and antitumor capacity. TCR, T cell receptor; CPT1, carnitine palmitoyltransferase 1; TCA, tricarboxylic acid cycle; acetyl-CoA, acetyl coenzyme A; FAO, fatty acid oxidation; FAS, fatty acid synthesis; OXPHOS, oxidative phosphorylation; PGC1α, peroxisome-proliferator-activated receptor gamma coactivator 1α.