Abstract
Over the last few decades, the Barents Sea experienced substantial warming, an expansion of relatively warm Atlantic water and a reduction in sea ice cover. This environmental change forces the entire Barents Sea ecosystem to adapt and restructure and therefore changes in pelagic–benthic coupling, organic matter sedimentation and long-term carbon sequestration are expected. Here we combine new and existing organic and inorganic geochemical surface sediment data from the western Barents Sea and show a clear link between the modern ecosystem structure, sea ice cover and the organic carbon and CaCO3 contents in Barents Sea surface sediments. Furthermore, we discuss the sources of total and reactive iron phases and evaluate the spatial distribution of organic carbon bound to reactive iron. Consistent with a recent global estimate we find that on average 21.0 ± 8.3 per cent of the total organic carbon is associated to reactive iron (fOC-FeR) in Barents Sea surface sediments. The spatial distribution of fOC-FeR, however, seems to be unrelated to sea ice cover, Atlantic water inflow or proximity to land. Future Arctic warming might, therefore, neither increase nor decrease the burial rates of iron-associated organic carbon. However, our results also imply that ongoing sea ice reduction and the associated alteration of vertical carbon fluxes might cause accompanied shifts in the Barents Sea surface sedimentary organic carbon content, which might result in overall reduced carbon sequestration in the future.
This article is part of the theme issue ‘The changing Arctic Ocean: consequences for biological communities, biogeochemical processes and ecosystem functioning’.
Keywords: Barents Sea, geochemical sediment composition, organic carbon bound to reactive iron, carbon cycle, Arctic Ocean, marine surface sediments
1. Introduction
One of the most apparent signs of current global climate change is Arctic sea ice loss. Over the past four decades, summer sea ice extent has drastically decreased by over 30% [1,2] and the ongoing transformation of the Arctic Ocean from an ‘icy land’ into an open ocean forces the entire Arctic ecosystem to adapt and restructure [3]. As the Arctic Barents Sea Shelf area (figure 1) is a transition zone between the temperate North Atlantic and the cold Arctic Ocean, it is climatically divided into two distinct regions. The northern area experiences a cold and harsh Arctic climate and sustains an ice-associated ecosystem, while the southern part has an Atlantic climate with a rich open water ecosystem and lucrative fisheries [4,5]. During recent decades, enhanced inflow of Atlantic water and atmospheric heat transport have dramatically warmed the Arctic, and in particular the Barents Sea [6]. Sea ice loss and ‘Atlantification’ of the northern Barents Sea are the consequences [6–8]. Higher water temperatures and sea ice reduction modifies the Arctic marine ecosystem structure and, therefore, changes the Arctic carbon cycle, i.e. atmospheric CO2 uptake, pelagic–benthic coupling, organic matter sedimentation and long-term sequestration [3,9–13]. An increase in the annual net primary production in the Arctic and the Barents Sea has already been observed since the late 1990s and might rise in the future, due to further summer sea ice reduction and longer phytoplankton growing seasons [10,14]. However, these environmental changes are complex and so far only a few studies link ongoing changes in the Arctic Ocean to organic carbon burial, sedimentary biogeochemical cycles and the marine ecosystems [11,15,16]. Thus, there is substantial uncertainty regarding current and future productivity and carbon burial estimates in the Arctic and the Barents Sea.
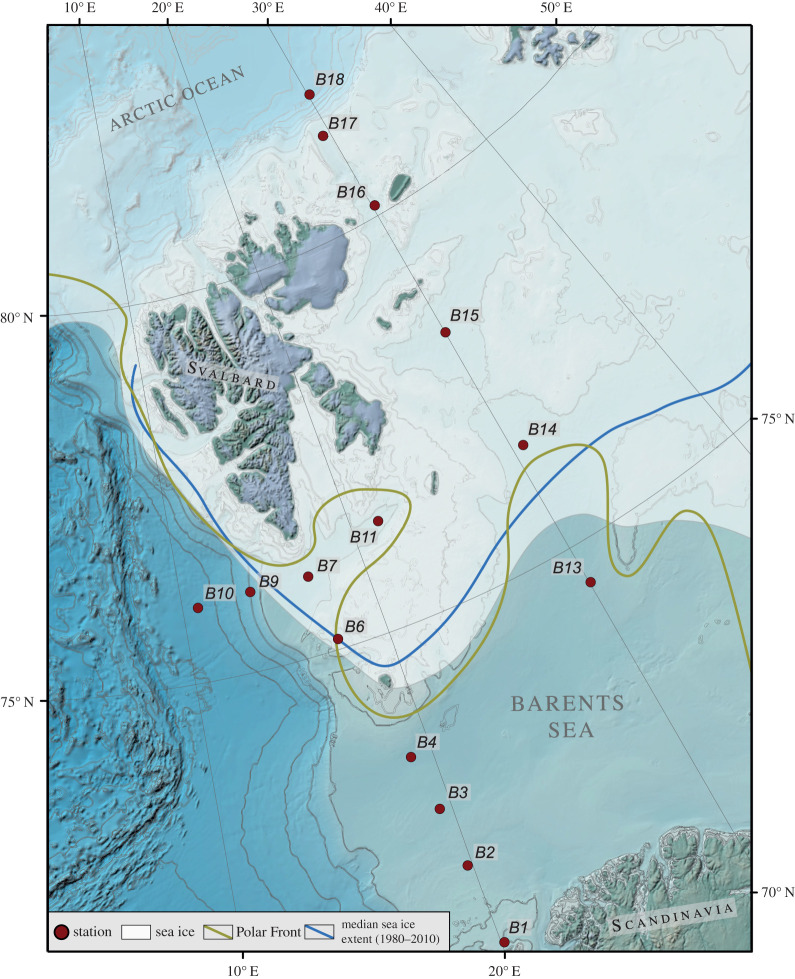
Figure 1.
Map of the western Barents Sea and sampling locations (red dots). The northern Barents Sea is seasonally ice-covered and winter maximum and median sea ice coverage over the past 40 years [2] are shown as white area and blue line, respectively. The boundary between the relatively warm northward flowing North Atlantic Current and the southward flowing cold Arctic currents forms the oceanographic Polar Front (yellow line). (Online version in colour.)
The sequestration of organic matter in marine sediments is a fundamental mechanism for the removal of carbon from the atmosphere and its storage over geological time periods [17]. Examining climatically induced biogeochemical changes in Arctic marine sediments, is therefore, important for a better understanding of the global carbon cycle. However, the processes that control organic carbon preservation in marine sediments, including sedimentation rate [18,19], presence and absence of oxygen [20–22], selective preservation of biochemically unreactive compounds [23,24], and protection of organic matter through interactions with a mineral matrix [25–27] are complex and still not fully understood. A possible connection between iron and organic carbon in marine sediments was already identified in 1970 [28], but only recently has the importance of this relationship for organic matter preservation in marine sediments been recognized [29]. Due to their high sorption capacity, iron oxides, in particular freshly precipitated and poorly crystalline iron (oxyhydr)oxides, like ferrihydrite, have a strong influence on organic carbon stabilization. During burial at the seafloor, organic carbon adsorbed to these oxides is preserved against microbial degradation and can therefore bypass the shallower oxic degradation regimes into, and possibly beyond, the zone of dissimilatory metal oxide reduction [29]. Therefore, reactive iron phases may serve as an efficient shuttle to enhance organic carbon burial and preservation in marine sediments. Lalonde et al. [29] investigated surface sediment samples from several marine environments including the Southern Ocean, Mexican and Indian Margins, St. Lawrence estuary and gulf, and the Black Sea. They proposed that on average 21.5% of the total organic carbon in marine surface sediments is associated with reactive iron globally. Hence, Lalonde et al. [29] stated that ‘reactive iron phases serve as an extremely efficient “rusty sink” for organic carbon and are a key factor in the long-term storage of organic carbon and the global cycles of carbon, oxygen and sulfur’. However, since this pioneering publication only a few studies have investigated the role of reactive iron on the preservation of organic carbon in natural marine sediments [30–37]. And except for one study from the East Siberian Arctic Shelf [30], the type and amount of organic carbon bound to iron oxides has not been examined in Arctic marine sediments. Moreover, there is still a general lack of knowledge about reactive iron sources in relation to total iron content, the general sediment composition, and the environmental setting. Making these mechanistic links is, however, necessary to evaluate the role of organic carbon bound to iron phases and its role in the global carbon cycle, especially in a fast-changing environment such as the Arctic Ocean.
To better understand how ongoing ‘Atlantification’ of the Barents Sea will change the organic and inorganic sediment composition in the future, we combined new and existing surface sediment (0–1 cm) data of organic carbon, total iron, calcium carbonate and grain size distribution of the seasonally ice-covered north and permanently ice-free south western Barents Sea. Furthermore, to better constrain the controls on, and efficiency of, carbon burial in the Arctic shelf seas we analysed the fraction of organic carbon bound to dithionite-extractable iron phases (fOC-FeR).
(a). Study area
The Barents Sea is located between 70–81°N off the northern Norwegian coast and is bordered by the shelf edge towards the Norwegian Sea in the west, the Norwegian archipelago Svalbard in the northwest and the islands of Franz Josef Land and Novaya Zemlya (Russia) in the northeast and east. It is the largest pan-Arctic shelf sea covering an area of 1.6 million square km with an average water depth of 230 m [38]. There are several extensive overviews and reviews about the modern climate setting and ecosystem of the Barents Sea and we refer to these references for a detailed description of the physical and ecological conditions [4,10,39–42]. In brief, the present ecological setting as in all Arctic seas is characterized by very pronounced seasonal fluctuations in insolation and, hence, primary production. However, despite the relatively short duration of the growing season in the Arctic, the Barents Sea is a high productivity shelf area where 40% of the total primary production of the Arctic Ocean takes place [43]. Water column primary productivity is generally inversely related to sea ice cover, i.e. lower rates occur in the northeast (30–70 g C m−2 yr−1) and higher and less variable rates in the Atlantic water-influenced southwest (100–150 g C m−2 yr−1) [39,44]. The general oceanic circulation pattern of the western Barents Sea is dominated by the relatively warm northward flowing North Atlantic Current (temperature 2–8°C, salinity greater than 35‰) which enters the Barents Sea from the southwest and the southward flowing cold Arctic currents (Spitsbergen and Persey; temperature less than 0°C, salinity less than 35‰) entering the Barents Sea from the northeast. The relatively sharp boundary between these water masses forms the oceanographic Polar Front (figure 1) [45] which is mainly determined by the bathymetry and is, therefore, relatively stable from year to year [46]. The northern Barents Sea is seasonally ice covered with maximum and minimum ice coverage in March–April and August–September, respectively. The heat content of the Atlantic water keeps the southern Barents Sea permanently ice-free. River runoff into the Barents Sea is very limited. Only one larger river, the Pechora River, enters directly into the south-eastern Barents Sea in Russia. Rivers on the Kola Peninsula, on Svalbard and in Norway are small and often drain into fjords. Thus, sediment discharge through river inflow is low and the main processes responsible for Barents Sea surface sediment distribution are re-deposition by winnowing from shallow banks into troughs and depressions, and deposition from sea ice. Hence, sedimentation rates are generally low, 0.04–2.1 mm yr−1 since the last glacial period, but can be much higher proximal to glacier outlets e.g. close to Svalbard (figure 2; electronic supplementary material, table S1).
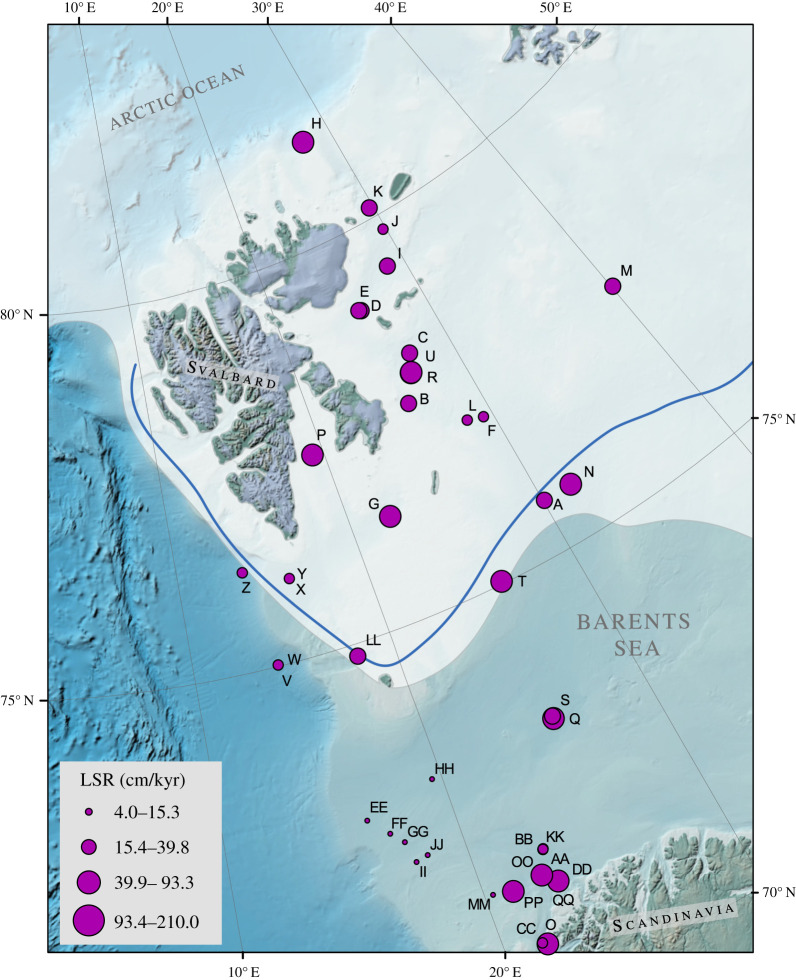
Figure 2.
Published linear sedimentation rates (LSR) in the Barents Sea. Data and references are provided in electronic supplementary material, table S1. (Online version in colour.)
2. Material and methods
(a). Surface sediments: sampling and preparation
In July 2017, surface sediment samples were collected using a multi-corer at 15 stations (electronic supplementary material, table S2) along a general south–north gradient in the western Barents Sea (figure 1). The first 1 cm of an undisturbed short sediment core at each station was sampled on-board the Royal Research Vessel James Clark Ross immediately after core recovery. At seven stations (B3, B13–B18) samples were taken in 0.5 cm intervals and all samples were stored in plastic bags at −20°C. Prior to any sediment analysis, except for grain size measurements, all samples were freeze-dried and homogenized by gentle grinding using an agate mortar and pestle.
(b). Bulk elemental composition and grain size analysis
Element composition of Barents Sea surface sediments was determined by wavelength dispersive X-ray fluorescence (XRF). A sample split of 700 mg was mixed with 4200 mg di-lithiumtetraborate (Li2B4O7, Spectromelt A10), preoxidized at 500°C with 1.0 g NH4NO3 (p.a.) and fused to homogeneous glass beads. The glass beads were analysed for 31 elements (Si, Ti, Al, Fe, Mn, Mg, Ca, Na, K, P, As, Ba, Co, Cr, Cu, Ni, Pb, Rb, Sr, V, Y, Zn, Zr) using a Philips PW-2400 WD-XRF spectrometer calibrated with 53 geostandards at the University of Oldenburg. Analytical precision and accuracy were better than 5% as checked by in-house and international reference materials. Results are provided in electronic supplementary material, table S3.
Grain size distribution was determined using a Mastersizer 2000E laser diffractometer at Leeds University, UK. Samples taken in 0.5 cm intervals (stations B3, B13–B18) were mixed prior to grain size analysis. Sediment samples were disaggregated in an ultrasonic bath for at least 15 min and grain size distribution of all samples were analysed on bulk and on decarbonated samples, which were treated with 10% (vol.) HCl before analysis. Grain size analysis was carried out on material within a particle diameter range of 0.1 to 1000 µm and results are presented as cumulative volume percentages (electronic supplementary material, tables S4 and S5).
(c). Organic carbon and reactive iron extraction and analysis (OC-Fe)
To quantify the amount of organic carbon bound to iron oxides in Barents Sea surface sediment samples we applied a citrate–dithionite iron reduction method which simultaneously dissolves all reactive iron (oxyhydr)oxides and the organic carbon associated with these phases (OC-Fe). A detailed description of the method can be found in Salvadó et al. [30]. Briefly, 0.25 g of each sample was transferred into 30 mL centrifuge tubes. 15 mL of a solution containing 0.27M trisodium citrate (Na3C6H5O7 · H2O) and 0.11M sodium bicarbonate (NaHCO3) was added, well mixed and heated up to 80°C in water bath. 0.1M sodium dithionite (Na2S2O4) was added to the mixture, maintained at 80°C and shaken every 5 min. After 15 min, the mixture was centrifuged for 10 min at 4000 rpm and the supernatant was decanted and 200 µl of HCl were added to prevent Fe precipitation. The sediment samples were rinsed three times with artificial seawater and then freeze-dried. To quantify the organic carbon loss during the experiment, which was unrelated to iron oxides dissolution, a control experiment was conducted. For the control experiment, a 0.25 g aliquot of each sample was treated the same way as for the reduction experiment but the complexing and reducing agents (sodium citrate and sodium dithionate) were replaced with sodium chloride to reach a solution of the same ionic strength. All samples were weighed after the experiment to account for mass loss during the experiment. Dissolved iron in the supernatant and rinse water of the control and reduction experiment was analysed using a Thermo Scientific iCE3000 Atomic Absorption Spectrometer (AAS) at Leeds University, UK. Results are shown in electronic supplementary material, table S6 and the relative error of the Fe analysis was ±2.6%.
Organic carbon (OC) content of the bulk sediment before and after the reduction and control experiments was analysed on decarbonated samples using 10% (vol.) HCl, rinsed three times and dried overnight at 50°C. OC content was determined with a LECO SC-144DR combustion analyser at Leeds University, UK (electronic supplementary material, table S6). The certified reference material LECO 502-062 and blanks were included in every batch, and results are given in weight percentage. The relative error of the OC analysis was ±1.7%.
(d). Sedimentary nitrogen and carbon isotope analysis
Freeze dried sediments (approx. 0.1 g) were acidified using 4 mol HCl (hydrochloric acid) to remove carbonates for 4 h, dried overnight at 60°C and analysed on a CS230 Carbon/Sulfur Determinator (Leco Corporation, Michigan, USA) using porous crucibles to derive total organic carbon content (TOC). Precision/reproducibility was ±<0.1%. Total carbon (TC) and nitrogen were determined on a VarioMAX CNS Analyser (Elementar, Langenselbold, Hesse, Germany) in at least duplicate (precision/reproducibility ±<0.1%). Total inorganic carbon (TIC) was calculated as the difference between the TC and TOC (TC-TOC). The calcium carbonate (CaCO3) content was estimated by multiplying TIC by 8.333. Bulk δ13Corg was analysed at Elemtex Laboratories (Cornwall, UK) using IRMS on samples acidified three times using 4 mol HCL with drying at 60°C between each acidification (precision/reproducibility to ±0.2‰).
3. Results and discussion
(a). Sources, spatial distribution and burial of organic carbon
Compared to organic carbon cycling processes in the water column, there is generally a lack of knowledge about the fate of sedimentary organic matter at and in the Arctic Barents Sea seafloor [47–50]. The link between vertical carbon export and accumulation to primary productivity patterns and terrestrial sources is still not well understood. Therefore, uncertainty remains about the origin of the sedimentary organic carbon, especially in the northern Barents Sea. Based on Corg/Ntot ratios, δ13Corg signatures and pigment analysis, several studies argue that the main source of sedimentary organic matter (OM) in Barents sea surface sediments is marine and derives from productivity in the water column and ice-associated algae production [16,47,51–54]. However, by accounting for the sedimentary inorganic nitrogen content, Knies et al. [55] showed that high amounts of terrigenous OM (≥50 rel. %) can be present in the seasonally sea ice covered and coastal regions of the northern Barents Sea, while high contributions of marine OM (>60 rel. %) occur in the ice-free southwestern Barents Sea. Our δ13Corg values from the northern station B13–B17 vary between −21.35‰ to −23.08‰ and Corg/Ntot values range in all stations between 6 and 8.5 (electronic supplementary material, table S3), which indicates that these locations are strongly influenced by marine OM.
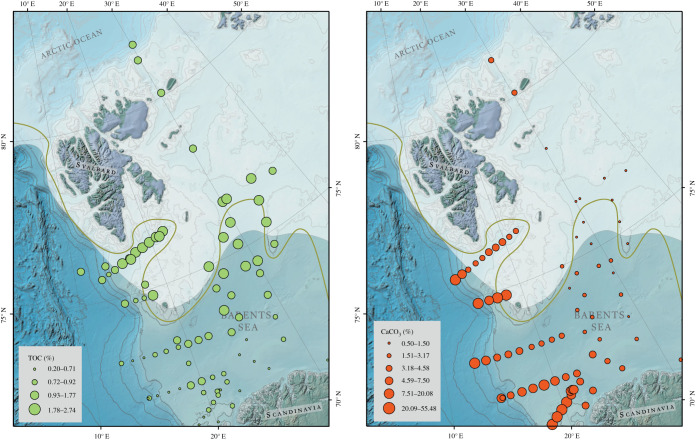
The total organic carbon (OC) content of the Barents Sea surface sediments from this study, as well as available OC data from the literature (figure 3) [47,50,56] show very similar trends. The OC content is higher in northern Barents Sea surface sediments and in coastal areas, whereas the ice-free southern areas show much lower OC contents (figure 3). Previous investigation of carbon burial rates in the northern Barents Sea show that carbon preservation in these sediments is considerably higher compared to other Arctic shelf areas [47]. A compilation of published linear sedimentation rates (figure 2; electronic supplementary material, table S1; adapted and extended from Pathirana et al. [50]) shows that sedimentation rates vary between 4 and 210 cm/kyr−1 (average 64 cm/kyr−1) for the entire western Barents Sea. They are lowest close to the western continental shelf edge, probably due to higher current velocities, and sedimentation rates in the seasonally ice covered northern Barents Sea (north of the median winter sea ice extent) are on average slightly higher (78.9 cm/kyr) than in the permanently ice-free southern regions (53.8 cm/kyr, south of the median winter sea ice extent, figure 2). This might be related to lower bottom current speed and higher sediment input from Svalbard and sea ice. The OC spatial distribution pattern could be related to different sedimentation rates and thus different oxygen exposure times as OC remineralization via oxygen reduction in marine sediments is the most effective process for OM degradation. However, investigations of sediment mixing and oxygen penetration depth of Barents Sea surface sediment show that at least the first centimetre is homogenized through physical and/or biological mixing [52,57] and that the oxygen penetration depth in most locations of the Barents Sea is greater than 1 cm [49,58]. Hence, we assume that the overall OC decomposition is comparable between the northern and southern Barents Sea and that the spatial distribution of OC between the northern and southern Barents Sea is related to other controlling factors. Hence, we used the average sedimentation rates to estimate the average carbon burial rates north and south of the median winter sea ice extent (electronic supplementary material, table S7). In the seasonally sea ice covered northern area organic carbon burial rates are (6.3 gC/m2 yr1) more than twice as high as in the ice-free southern region (2.4 gC/m2 yr1). Even though these numbers present only an approximation derived from surface sedimentary OC, they are in relatively good agreement with carbon accumulation rate of 5.5 gC/m2 yr1 published previously for the northern Barents Sea area [47]. Based on these findings, we suggest that carbon sequestration in the ice-free southern Barents Sea sediments is lower compared to the ice-covered northern region.
Figure 3.
Spatial distribution of CaCO3 (left) and total organic carbon (right) in Barents Sea surface sediments. For further legend details see figure 1. (Online version in colour.)
(b). Inverse relationship between total organic carbon content and calcium carbonate
In pelagic sediments, variations in biogenic carbonate content are mainly controlled by dissolution, dilution, and/or productivity changes. Hence, due to the strong relationship of CaCO3 to marine productivity and, thus, water temperature, salinity, nutrient supply and degree of ice coverage, CaCO3 is often applied as a proxy to reconstruct climate and environmental changes. Carbonate content in surface sediments from the eastern central Arctic Ocean, north of the Barents Sea, were found to be mainly of biogenic origin [59] and CaCO3 contents in southern Barents Sea surface sediments show a good correspondence with planktonic foraminifera abundances [60]. In agreement with these findings, our results show a strong relationship between CaCO3 and Ca (r = 0.99) and both parameters are anti-correlated to terrigenous elements like Si, Fe, K, Ti and Al (r ≤ −0.49; electronic supplementary material, figure S1). This suggests that the carbonate content in Barents Sea sediments largely reflects the calcareous shell fragments from either planktonic or benthic organisms and, that terrigenous CaCO3 sources have only a very minor effect on the composition of Barents Sea surface sediments.
The variable carbonate content is also reflected in the grain size distribution in Barents Sea surface sediments (figure 4). In the southern Barents Sea, bulk grain size distribution at stations B1 to B11 is much more heterogeneous with higher contributions of coarse-grained material (35% >63 µm) compared to the clay and silt fraction dominated northern stations B13 to B18 (87% <63 µm). The decarbonated grain size analyses, however, show that the siliciclastic fraction is dominated by the silt fraction (average 81%) and very homogeneously distributed in Barents Sea surface sediments (figure 4). This shows that the bulk grain size measurements of Barents Sea sediments are strongly modulated by their carbonate content.
Figure 4.
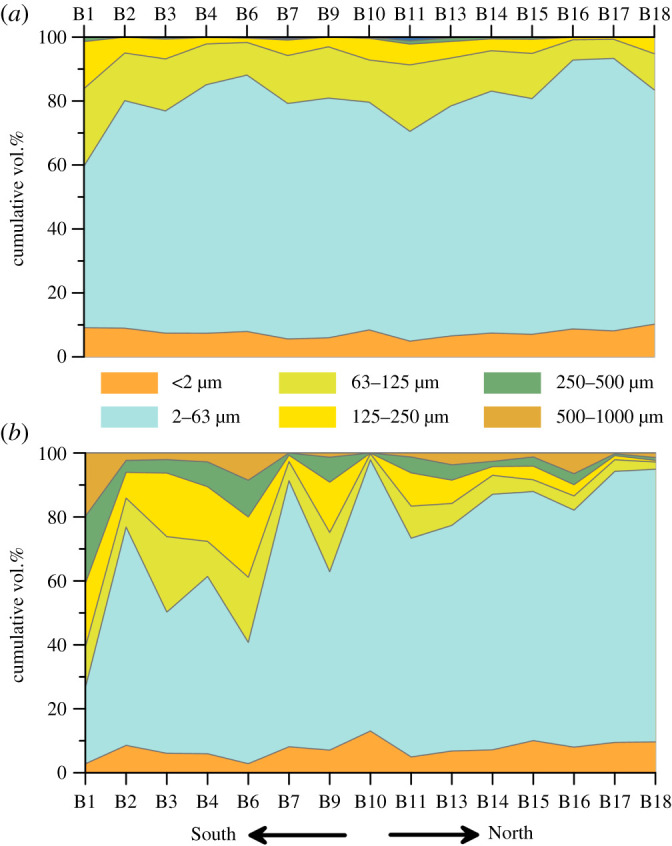
Grain size distribution in Barents Sea surface sediments in (a) decarbonated and (b) bulk sediment samples. (Online version in colour.)
Since CaCO3 in Barents Sea surface sediments is assumed to be mainly of marine origin, higher CaCO3 content indicates higher primary productivity, which could be expected to result in higher organic matter fluxes towards the seafloor. But the CaCO3 content in Barents Sea surface sediments shows an opposite pattern to the OC distribution, i.e. low OC content in the south-western part coincides with high CaCO3 content, and vice versa in the north-eastern part (figure 3) [56]. A possible reason could be OC dilution through higher CaCO3 contents in the south-western area of the Barents Sea. However, a calculation of OC contents on a CaCO3 basis (see electronic supplementary material, figure S2) does not indicate a strong dilution effect of OC through inorganic carbon. Moreover, in the very productive Storfjord trough south of Svalbard (Station B7, B9–B11), both OC and CaCO3 show relatively high concentrations. Steinsund et al. [60] attributed differences in the CaCO3 content to carbonate dissolution in the north-eastern Barents Sea caused by dense, cold, saline and CO2-rich bottom water produced by sea ice formation. However, while this may explain the lower carbonate content north of the polar front, it cannot explain the described regional differences in the OC content, since OC is not susceptible to dissolution by CO2-rich waters. Moreover, dense cold bottom water currents produced by sea ice brine formation also occur in areas where CaCO3 concentrations are high, for example in the Storfjord trough (Station B7, B9–B11) [61–63]. Hebbeln et al. [64] showed that the carbonate content in the surface sediments of the Polar North Atlantic reflect the influx of temperate Atlantic waters into the Nordic Seas, where the highest carbonate content follows the main axis of the Norwegian Current and decreases with lower water temperature northwards and to the west. Moreover, sea ice cover reconstruction based on a sediment core from the south-western Barents Sea showed that seasonal sea ice cover during the early Holocene was accompanied by lower carbonate content and a clear increase in the total sedimentary organic carbon concentrations [65]. These findings indicate that low carbonate content in the north-east Barents Sea is likely related to cold Arctic [39] water masses, with lower carbonate production, while higher CaCO3 content in the south-western Barents Sea sediments are probably related to the warmer Atlantic water inflow (figure 3). Hence, we suggest that the opposite distribution pattern of OC and CaCO3 in the seasonally sea ice-covered north-western Barents Sea and the ice-free southern area (figure 3) could be related to differences in primary productivity and vertical OM flux rates. Wassmann et al. ([66] and references therein) showed that the main phytoplankton bloom development occurs in May/June in the southern Barents Sea and is relatively predictable. The spring bloom in the northern Barents Sea, however, depends on the sea ice conditions which are highly variable, and the bloom develops more rapidly than in the southern Barents Sea. It follows that while predators are well-adapted to the spring bloom in the southern Barents Sea, the rapid and unpredictable development of the spring bloom in the marginal ice zone typically decouples phytoplankton development from zooplankton grazing [39]. Thus, despite the ice cover, OC pelagic–benthic fluxes are probably higher in the northern Barents Sea due to lower OM consumption in the water column. Additionally, the export of ice algae (diatoms) might substantially contribute to high OM export fluxes in the marginal ice zone [67]. Beyond OM export quantity, high pelagic consumption and recycling also reduces the quality of vertically exported OM, while low to moderate pelagic consumption allows OM of higher quality to reach the seafloor [68]. In accordance with investigations of the pelagic–benthic coupling and related OM fluxes from the water column to the seabed in the Arctic and Northeast Atlantic [9,66] we suggest that the increased sedimentary OC contents in the northern Barents Sea (figure 3) are related to higher rates of OC delivery to the seafloor. This trend in OM export appears to be matched by similar trends in the benthic macro- and megafauna. A clear and consistent south–north distribution pattern of benthic organisms with generally more taxa, higher biomass and higher abundance in the northern Barents Sea implies increased OM fluxes, which support the benthic ecosystem [40]. If we use the environmental setting of the southern ice free Barents Sea as an analogue for a future ice free northern Barents Sea, these findings imply that with ongoing climate change, the northern Barents Sea may transform from a cold and stratified Arctic to a southern Barents Sea-like warm and well-mixed Atlantic-dominated climate regime [6]. This change may lead to a shift from the current ‘sea ice algae–benthos' ecosystem to a ‘phytoplankton–zooplankton’ dominated ecosystem [9]. Since our findings indicate a link between marine productivity and the geochemical composition of Barents Sea surface sediments, ongoing sea ice reduction and the associated alteration of pelagic primary productivity are expected to cause accompanied shifts in the Barents Sea surface sediment composition. Compared to the modern situation, the northern Barents Sea surface sediments might contain higher contents of CaCO3 and less OC, which could result in reduced OC burial rates in the future.
(c). Preservation of organic matter promoted by iron in Barents Sea surface sediments
To evaluate the preservation of OC in the seasonally ice covered northern Barents Sea and the ice-free southern area, we determined the amount of organic carbon associated with reactive iron phases by applying a citrate–dithionite iron reduction method [29]. In the following, we will discuss the sources of total and reactive iron in Barents Sea surface sediments. Thereafter, we evaluate the spatial distribution pattern of OC bound to iron and show that the fraction of total organic carbon bound to reactive iron phases is not related to sea ice cover.
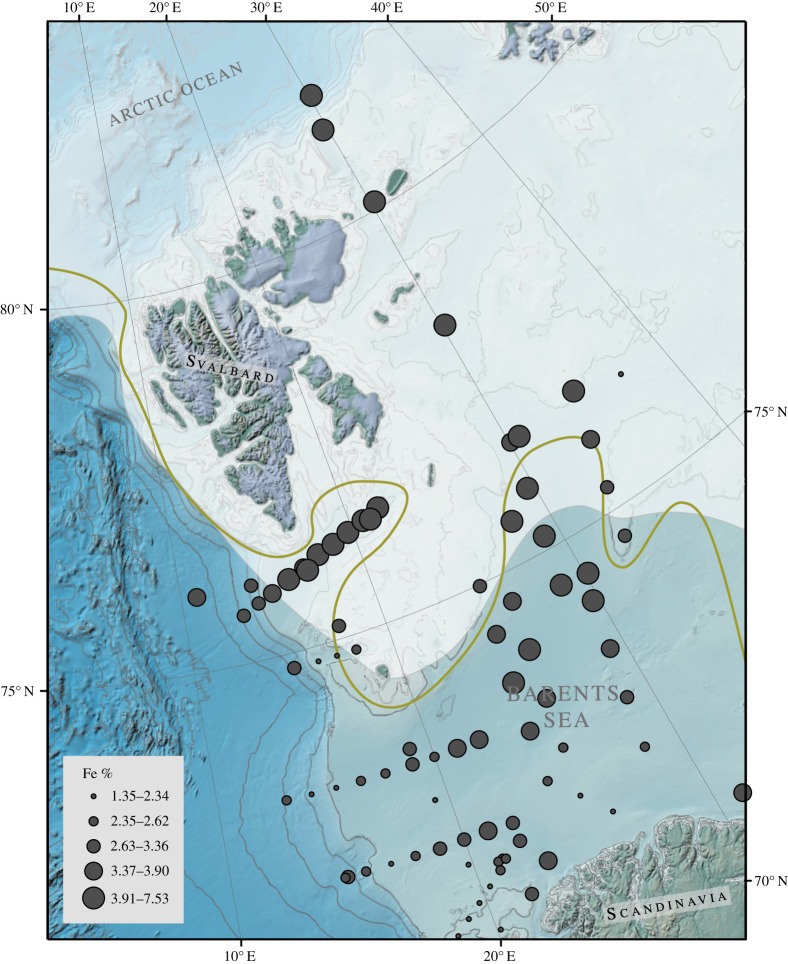
In accordance with the previously published spatial distribution pattern of iron in surface sediments from the southern Barents Sea, our results show that the bulk iron contents in Barents Sea surface sediments are highest to the eastern side of the Svalbard archipelago (stations B14–B18) (figures 5 and 6b; Knies et al. [56]). Values decrease towards the south with intermediate concentrations south of Svalbard (station B9–B13), and lowest values in the south-western Barents Sea (stations B1–B6). Higher iron contents in northern Barents Sea sediments are probably related to bedrock erosion by glaciers on Svalbard [69–71], deposition from sea ice [72,73] and erosion of Barents Sea Mesozoic bedrock [71,74]. Our results show that the reactive iron (FeR) abundance is strongly related to the sedimentary bulk iron content (r = 0.94, n = 22, electronic supplementary material, figure S3). Thus, the FeR contents and the relative contributions of dithionite-extractable reactive iron oxides show a south–north gradient as well (figure 6c,d). The reactive iron fraction of the total iron content (fFeR) in samples from the south-western stations B1–B13 is on average 16.2%, whereas fFeR contents in samples north of the Polar Front (B14–18) are on average 27.9%. Thus, as sediment samples from seasonally sea ice covered stations contain the highest OC content and show highest fFeR contribution (figure 6a,d) we would expect them to have a high potential to bind OC to iron oxides as well. Indeed, we find that the amount of OC bound to iron (OC-FeR) is on average about three times higher in the northern Barents Sea compared to the south-western area (figure 6e). The strong relationship between FeR and OC-FeR is in accordance with Ma et al. [34] who investigated literature data of OC-FeR and suggest that OC-FeR contents in marine surface sediments are highly dependent on OC and FeR availability. Moreover, our data show no clear spatial relation between sea ice cover and OC-FeR content. Stations B6, B7 and B11 were affected by winter sea ice at least for the past 40 years (figure 1) [2]. But compared to B11, OC-FeR concentrations at B6 and B7 are very low. B13 is not affected by sea ice but OC-FeR concentrations are high (figure 6e). This implies that sea ice cover has no direct impact on the preservation of OC through FeR sorption.
Figure 5.
Spatial distribution of iron in Barents Sea surface sediments. Data from this study and Knies et al. [56]. (Online version in colour.)
Figure 6.
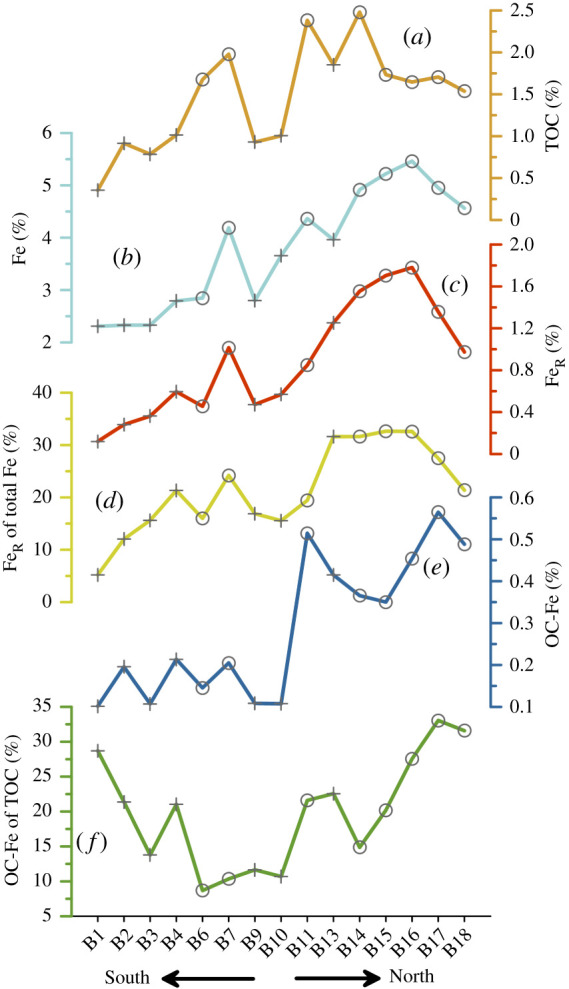
Distribution of (a) TOC, (b) bulk Fe, (c) reactive ion, (d) reactive iron fraction of total iron (fFeR), (e) organic carbon bound to reactive iron (OC-FeR) and (f) the organic carbon fraction of total organic carbon bound to reactive iron (fOC-FeR) in Barents Sea surface sediments (0–1 cm). Circles mark stations which are seasonally sea ice covered and crosses are stations which are ice free during winter. Station locations (B1–B18) and ice coverage is shown in figure 1. (Online version in colour.)
In contrast to OC-FeR, the spatial distribution of the OC fraction of the total sedimentary OC pool bound to FeR (fOC-FeR) (figure 6f) shows no relationship to either TOC or FeR contents and, therefore, does not show a spatial south–north gradient. Also, an association to sea ice cover, proximity to land, grain size distribution or sediment composition were not identified either. In fact, the fraction of OC bound to FeR in the southern Barents Sea is very similar to that in the northern Barents Sea region (figure 6f), even though sample locations are very different in terms of their environmental settings, sediment sources, OC and FeR contents (see discussion above). Thus, a relatively high fraction of OC can be bound to FeR even if absolute FeR contents are relatively low. This suggests that the amount of OC bound to reactive iron is not dependent on the total amount of FeR available, but that other factors such as the organic matter type and composition as well as redox processes play an important role. This assumption is in accordance with findings from the Eurasian Arctic Shelf. Salvadó et al. [30] showed that the composition of the OC associated with the Fe phases changes with the OM source (i.e. marine versus terrigenous), and that in Arctic shelf areas dominated by marine OM, fOC-FeR can be lower than in areas dominated by remobilized terrigenous OC, e.g. from thawing permafrost. Also Zhao et al. [31] found that in estuarine sediments in southern China, FeR was largely associated with terrigenous OC. Moreover, the association between OC and FeR is formed mainly through co-precipitation/chelation and/or adsorption [29,33,75]. Coprecipitation has a higher sorption capacity of OC and occurs when upward diffusing pore water Fe2+ is oxidized at the redox interface in the presence of dissolved OC. Thus, it has been proposed that Fe redox processes are ‘ultimately the overarching determinant’ of fOC-FeR in marine sediments [34]. Even though most observations suggest that the oxygen penetration depth in the Barents Sea is greater than 1 cm [49,58] and that the first centimetre of Barents Sea surface sediments is affected and homogenized through physical and/or biological mixing [52,57], the redox interface might still reach into the first centimetre, e.g. due to high Fe2+ upward fluxes or seasonal changes of the oxygen penetration depth through primary productivity variability. At seven stations (B3, B13–B18) we analysed the OC bound to iron in 0.5 cm depth intervals. The results show no significant differences between the TOC, Fe and FeR contents in the 0–0.5 cm and 0.5–1 cm sections (electronic supplementary material, figure S4), confirming that the first centimetre is well mixed. Compared to the 0.5–1 cm section, fFeR, OC-FeR and fOC-FeR contents are in general slightly higher in the first half centimetre. This implies that the effect of redox processes (Fe2+ upward fluxes) on the fOC-FeR content in the first centimetre of Barents Sea sediments is minor.
Besides the investigation of natural samples, recent experimental laboratory studies on the composition of FeR-associated organic matter revealed that varying OCF:FeR molar ratios are related to the binding mechanism of OC with FeR phases: adsorption results in lower OCF:FeR ratios (less than or equal to 1), while co-precipitation yields ratios between 6 and 10 [76]. In turn, the impact of adsorption and co-precipitation on organic matter loadings ultimately depends on the organic matter composition and redox processes [33,75]. In Barents Sea surface sediments, OCF:FeR molar ratios vary between 0.9 and 3.8 (average = 1.8) and are in the range for sediments overlain by oxic bottom waters [29] (electronic supplementary material, table S6 and figure S5). The majority of OCF:FeR values show only small variations between about 1–2; only stations B1, B2 and B11 show relatively high values of 2.9, 3.3 and 3.8, respectively. This might indicate that besides the large differences in the biogeochemical characteristics of the Barents Sea shelf regimes, the composition of OC bound to FeR is relatively similar, maybe due to generally low contributions of terrigenous OM at all investigated locations [77]. However, OCF:FeR values of stations B3 and B14–B18 show average values of 1.6 and 1.9 for the upper and lower half centimetre, respectively (electronic supplementary material, table S6). This indicates that the effect of coprecipitation is either very small or that factors other than the binding mechanisms of OC to Fe oxides, such as mineralogy or Fe-oxide reactivity influence the OCF:FeR ratio. Moreover, competitive sorption by arsenic (As) or phosphorus species onto Fe oxide surfaces, can influence the OCF:FeR ratio. For example, As contents in our Barents Sea samples are strongly related to FeR contents (r = 0.9, n = 15) but show a weak correlation with fOC-FeR (r = 0.5, n = 15), hence it is likely that surface sorption sites on Fe oxides can be ‘blocked’ by As and thus are unavailable for OC binding. To further evaluate differences in the OCF:FeR ratios in natural sediments from the Barents Sea and globally, we need to develop a better understanding of the composition and type of the organic matter bound to iron oxides and the timing of when this bonding occurs.
4. Implications and conclusion
Strong regional differences in the surface sediment composition between the northern, seasonally sea ice-covered and the southern, ice-free region of the western Barents Sea reveal that CaCO3 content shows an opposite pattern to the OC distribution, i.e. low OC content in the south-western part coincide with high CaCO3 content, and vice versa in the north-eastern part. We propose that this is likely related to the modern ecosystem structure with higher primary productivity but lower vertical organic carbon flux rates in the southern than in the northern Barents Sea. Low CaCO3 content in the north-east Barents Sea might be related to cold Arctic water masses, with lower carbonate production, while higher CaCO3 content in the south-western Barents Sea sediments is probably related to the warmer Atlantic water inflow.
Arctic warming will result in higher water temperatures, increased river run-off and reduced sea ice cover. Thus, the northern Barents Sea may transform from a cold and stratified Arctic to a southern Barents Sea-like warm and well-mixed Atlantic-dominated climate regime. This enormous environmental change will certainly induce substantial marine ecosystem changes. More extensive open water conditions and enhanced nutrient inputs through rivers are expected to enhance primary productivity. However, less sea ice cover in the northern Barents Sea may also lead to a shift of the typical ‘sea-ice algae–benthos’ ecosystem to a ‘phytoplankton–zooplankton’ dominated ecosystem. The proposed link between marine productivity and the geochemical composition of Barents Sea surface sediments implies that ongoing ‘Atlantification’ of the Barents Sea will affect the Barents Sea surface sediment composition and that compared to the modern situation the northern Barents Sea surface sediments might contain higher contents of CaCO3 and less OC in the future. Thus, a rise in primary productivity may lead to higher atmospheric CO2 uptake but higher carbon turnover rates/remineralization in the water column may decrease vertical OC fluxes in the northern Barents Sea.
To better constrain the controls on, and efficiency of, carbon burial in the Arctic shelf seas, we analysed the fraction of organic carbon bound to dithionite-extractable iron phases (fOC-FeR). Consistent with the global estimate by Lalonde et al. [29] 21% of the total organic carbon is on average associated to iron in Barents Sea surface sediments. We found that a relatively high fraction of OC can be bound to reactive iron even if absolute reactive iron contents are relatively low. Moreover, our findings indicate that the amount of OC bound to reactive iron is not dependent on the total amount of reactive iron available, but that the organic matter type and composition seem to be important factors in natural sediments. Furthermore, the spatial distribution of the organic carbon bound to iron seems to be unrelated to sea ice cover, Atlantic water inflow proximity to land, grain size distribution or sediment composition. Future Arctic warming might therefore neither enhance nor decrease carbon burial through the adsorption to iron oxides.
Supplementary Material
Supplementary Material
Acknowledgements
We thank the crew of the RRS James Clark Ross for their professional support during our expedition. Further, we would like to express our gratitude to Andy Connelly, Andrew Hobson, Fiona Keay, Gareth Keevil, Carola Lehners, Corinna Mori and Bernhard Schnetger for their help with the laboratory work at the University of Leeds and at the ICBM Oldenburg. We are grateful for the comments of two anonymous reviewers, which helped to improve the manuscript.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
J.C.F. was the lead author and wrote the manuscript. J.C.F., M.A.S., A.T. and C.M. conducted fieldwork/sampling together and compiled datasets. J.C.F., M.A.S., A.F., I.M., G.D.A., R.H. and J.P. carried out all the required analytical work and J.K. provided organic and inorganic elemental data. All authors contributed early ideas, revised the initial manuscript and provided a lively discussion.
Competing interests
We declare we have no competing interests.
Funding
This work resulted from the ChAOS project (grant no. NE/P006493/1 and NE/P00637X/1), part of the Changing Arctic Ocean programme, jointly funded by the UKRI Natural Environment Research Council (NERC) and the German Federal Ministry of Education and Research (BMBF). J.K. was funded by the Research Council of Norway (grant no. 223259).
References
- 1.Meier WN, et al. 2014. Arctic sea ice in transformation: a review of recent observed changes and impacts on biology and human activity. Rev. Geophys. 52, 185–217. ( 10.1002/2013RG000431) [DOI] [Google Scholar]
- 2.Fetterer F, Knowles K, Meier WN, Savoie M, Windnagel AK. 2017. Sea Ice Index, Version 3. Boulder, CO: NSIDC: National Snow and Ice Data Center. [Google Scholar]
- 3.Post E, et al. 2013. Ecological consequences of sea-ice decline. Science 341, 519–524. ( 10.1126/science.1235225) [DOI] [PubMed] [Google Scholar]
- 4.Smedsrud LH, et al. 2013. The role of the Barents Sea in the Arctic climate system. Rev. Geophys. 51, 415–449. ( 10.1002/rog.20017) [DOI] [Google Scholar]
- 5.Loeng H. 1991. Features of the physical oceanographic conditions of the Barents Sea. Polar Res. 10, 5–18. ( 10.3402/polar.v10i1.6723) [DOI] [Google Scholar]
- 6.Lind S, Ingvaldsen RB, Furevik T. 2018. Arctic warming hotspot in the northern Barents Sea linked to declining sea-ice import. Nat. Clim. Change 8, 634–639. ( 10.1038/s41558-018-0205-y) [DOI] [Google Scholar]
- 7.Polyakov IV, et al. 2017. Greater role for Atlantic inflows on sea-ice loss in the Eurasian Basin of the Arctic Ocean. Science 356, 285–291. ( 10.1126/science.aai8204) [DOI] [PubMed] [Google Scholar]
- 8.Barton BI, Lenn Y-D, Lique C. 2018. Observed Atlantification of the Barents Sea causes the Polar Front to limit the expansion of winter sea ice. J. Phys. Oceanogr. 48, 1849–1866. ( 10.1175/JPO-D-18-0003.1) [DOI] [Google Scholar]
- 9.Piepenburg D. 2005. Recent research on Arctic benthos: common notions need to be revised. Polar Biol. 28, 733–755. ( 10.1007/s00300-005-0013-5) [DOI] [Google Scholar]
- 10.Dalpadado P, et al. 2014. Productivity in the Barents Sea—response to recent climate variability. PLoS ONE 9, e95273 ( 10.1371/journal.pone.0095273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wassmann P. 2011. Arctic marine ecosystems in an era of rapid climate change. Prog. Oceanogr. 90, 1–17. ( 10.1016/j.pocean.2011.02.002) [DOI] [Google Scholar]
- 12.Wassmann P, Carroll J, Bellerby RGJ. 2008. Carbon flux and ecosystem feedback in the northern Barents Sea in an era of climate change: an introduction. Deep Sea Res. Part II Top. Stud. Oceanogr. 55, 2143–2153. ( 10.1016/j.dsr2.2008.05.025) [DOI] [Google Scholar]
- 13.Arrigo KR, van Dijken GL.. 2011. Secular trends in Arctic Ocean net primary production. J. Geophys. Res. 116. [Google Scholar]
- 14.Arrigo KR, van Dijken G, Pabi S.. 2008. Impact of a shrinking Arctic ice cover on marine primary production. Geophys. Res. Lett. 35. [Google Scholar]
- 15.Haug T, et al. 2017. Future harvest of living resources in the Arctic Ocean north of the Nordic and Barents Seas: a review of possibilities and constraints. Fish. Res. 188, 38–57. ( 10.1016/j.fishres.2016.12.002) [DOI] [Google Scholar]
- 16.Stein R, MacDonald RW. 2004. The organic carbon cycle in the Arctic Ocean. Berlin, Germany: Springer. [Google Scholar]
- 17.Berner RA. 2003. The long-term carbon cycle, fossil fuels and atmospheric composition. Nature 426, 323–326. ( 10.1038/nature02131) [DOI] [PubMed] [Google Scholar]
- 18.Müller PJ, Suess E. 1979. Productivity, sedimentation rate, and sedimentary organic matter in the oceans—I. Organic carbon preservation. Deep Sea Res. A 26, 1347–1362. ( 10.1016/0198-0149(79)90003-7) [DOI] [Google Scholar]
- 19.Ingall ED, Vancappellen P. 1990. Relation between sedimentation rate and burial of organic phosphorus and organic carbon in marine sediments. Geochim. Cosmochim. Acta 54, 373–386. ( 10.1016/0016-7037(90)90326-G) [DOI] [Google Scholar]
- 20.Canfield DE. 1994. Factors influencing organic carbon preservation in marine sediments. Chem. Geol. 114, 315–329. ( 10.1016/0009-2541(94)90061-2) [DOI] [PubMed] [Google Scholar]
- 21.Pedersen T, Calvert SE. 1990. Anoxia vs. productivity: what controls the formation of organic-carbon-rich sediments and sedimentary rocks? Am. Assoc. Pet. Geol. Bull. 74, 454–466. [Google Scholar]
- 22.Hartnett HE, Keil RG, Hedges JI, Devol AH. 1998. Influence of oxygen exposure time on organic carbon preservation in continental margin sediments. Nature 391, 572–574. ( 10.1038/35351) [DOI] [Google Scholar]
- 23.Burdige DJ. 2007. Preservation of organic matter in marine sediments: controls, mechanisms, and an imbalance in sediment organic carbon budgets? Chem. Rev. 107, 467–485. ( 10.1021/cr050347q) [DOI] [PubMed] [Google Scholar]
- 24.Hatcher PG, Spiker EC, Szeverenyi NM, Maciel GE. 1983. Selective preservation and origin of petroleum-forming aquatic kerogen. Nature 305, 498–501. ( 10.1038/305498a0) [DOI] [Google Scholar]
- 25.Hedges JI, Keil RG. 1995. Sedimentary organic matter preservation: an assessment and speculative synthesis. Mar. Chem. 49, 81–115. ( 10.1016/0304-4203(95)00008-F) [DOI] [Google Scholar]
- 26.Mayer LM. 1994. Relationships between mineral surfaces and organic-carbon concentrations in soils and sediments. Chem. Geol. 114, 347–363. ( 10.1016/0009-2541(94)90063-9) [DOI] [Google Scholar]
- 27.Hemingway JD, Rothman DH, Grant KE, Rosengard SZ, Eglinton TI, Derry LA, Galy VV. 2019. Mineral protection regulates long-term global preservation of natural organic carbon. Nature 570, 228–231. ( 10.1038/s41586-019-1280-6) [DOI] [PubMed] [Google Scholar]
- 28.Berner RA. 1970. Sedimentary pyrite formation. Am. J. Sci. 268, 1 ( 10.2475/ajs.268.1.1) [DOI] [Google Scholar]
- 29.Lalonde K, Mucci A, Ouellet A, Gelinas Y. 2012. Preservation of organic matter in sediments promoted by iron. Nature 483, 198–200. ( 10.1038/nature10855) [DOI] [PubMed] [Google Scholar]
- 30.Salvadó JA, Tesi T, Andersson A, Ingri J, Dudarev OV, Semiletov IP, Gustafsson A. 2015. Organic carbon remobilized from thawing permafrost is resequestered by reactive iron on the Eurasian Arctic Shelf. Geophys. Res. Lett. 42, 8122–8130. ( 10.1002/2015GL066058) [DOI] [Google Scholar]
- 31.Zhao B, et al. 2018. The role of reactive iron in the preservation of terrestrial organic carbon in estuarine sediments. J. Geophys. Res. Biogeosci. 123, 3556–3569. ( 10.1029/2018JG004649) [DOI] [Google Scholar]
- 32.Barber A, et al. 2017. Preservation of organic matter in marine sediments by inner-sphere interactions with reactive iron. Sci. Rep. 7, 366 ( 10.1038/s41598-017-00494-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shields MR, Bianchi TS, Gélinas Y, Allison MA, Twilley RR. 2016. Enhanced terrestrial carbon preservation promoted by reactive iron in deltaic sediments. Geophys. Res. Lett. 43, 1149–1157. ( 10.1002/2015GL067388) [DOI] [Google Scholar]
- 34.Ma W-W, Zhu M-X, Yang G-P, Li T. 2018. Iron geochemistry and organic carbon preservation by iron (oxyhydr)oxides in surface sediments of the East China Sea and the south Yellow Sea. J. Mar. Syst. 178, 62–74. ( 10.1016/j.jmarsys.2017.10.009) [DOI] [Google Scholar]
- 35.Linkhorst A, Dittmar T, Waska H. 2017. Molecular fractionation of dissolved organic matter in a shallow subterranean estuary: the role of the iron curtain. Environ. Sci. Technol. 51, 1312–1320. ( 10.1021/acs.est.6b03608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sirois M, Couturier M, Barber A, Gélinas Y, Chaillou G. 2018. Interactions between iron and organic carbon in a sandy beach subterranean estuary. Mar. Chem. 202, 86–96. ( 10.1016/j.marchem.2018.02.004) [DOI] [Google Scholar]
- 37.Wang D, Zhu MX, Yang GP, Ma WW. 2019. Reactive iron and iron-bound organic carbon in surface sediments of the river-dominated Bohai Sea (China) versus the southern Yellow Sea. J. Geophys. Res. Biogeosci. 124, 79–98. ( 10.1029/2018JG004722) [DOI] [Google Scholar]
- 38.Carmack E, Barber D, Christensen J, Macdonald R, Rudels B, Sakshaug E. 2006. Climate variability and physical forcing of the food webs and the carbon budget on panarctic shelves. Prog. Oceanogr. 71, 145–181. ( 10.1016/j.pocean.2006.10.005) [DOI] [Google Scholar]
- 39.Wassmann P, et al. 2006. Food webs and carbon flux in the Barents Sea. Prog. Oceanogr. 71, 232–287. ( 10.1016/j.pocean.2006.10.003) [DOI] [Google Scholar]
- 40.Jørgensen LL, Ljubin P, Skjoldal HR, Ingvaldsen RB, Anisimova N, Manushin I. 2015. Distribution of benthic megafauna in the Barents Sea: baseline for an ecosystem approach to management. ICES J. Mar. Sci. 72, 595–613. ( 10.1093/icesjms/fsu106) [DOI] [Google Scholar]
- 41.Loeng H, Ozhigin V, Adlandsvik B. 1997. Water fluxes through the Barents Sea. ICES J. Mar. Sci. 54, 310–317. ( 10.1006/jmsc.1996.0165) [DOI] [Google Scholar]
- 42.Jakobsen T, Ozhigin VK. 2011. The Barents Sea—ecosystem, resources, management. Half a century of Russian-Norwegian cooperation. Bergen, Norway: Tapir Akademisk Forlag. [Google Scholar]
- 43.Sakshaug E. 2004. Primary and secondary production in the Arctic seas. In The organic carbon cycle in the Arctic Ocean (eds Stein R, MacDonald RW), pp. 57–81. Berlin, Germany: Springer. [Google Scholar]
- 44.Eriksen E, Skjoldal HR, Gjøsæter H, Primicerio R. 2017. Spatial and temporal changes in the Barents Sea pelagic compartment during the recent warming. Prog. Oceanogr. 151, 206–226. ( 10.1016/j.pocean.2016.12.009) [DOI] [Google Scholar]
- 45.Harris CL, Plueddemann AJ, Gawarkiewicz GG. 1998. Water mass distribution and polar front structure in the western Barents Sea. J. Geophys. Res. Oceans 103, 2905–2917. ( 10.1029/97JC02790) [DOI] [Google Scholar]
- 46.Drinkwater KF. 2011. The influence of climate variability and change on the ecosystems of the Barents Sea and adjacent waters: review and synthesis of recent studies from the NESSAS Project. Prog. Oceanogr. 90, 47–61. ( 10.1016/j.pocean.2011.02.006) [DOI] [Google Scholar]
- 47.Carroll J, Zaborska A, Papucci C, Schirone A, Carroll ML, Pempkowiak J. 2008. Accumulation of organic carbon in western Barents Sea sediments. Deep Sea Res. Part II Top. Stud. Oceanogr. 55, 2361–2371. ( 10.1016/j.dsr2.2008.05.005) [DOI] [Google Scholar]
- 48.Nickel M, Vandieken V, Brüchert V, Jørgensen BB. 2008. Microbial Mn(IV) and Fe(III) reduction in northern Barents Sea sediments under different conditions of ice cover and organic carbon deposition. Deep Sea Res. Part II Top. Stud. Oceanogr. 55, 2390–2398. ( 10.1016/j.dsr2.2008.05.003) [DOI] [Google Scholar]
- 49.Vandieken V, Nickel M, Jorgensen BB. 2006. Carbon mineralization in Arctic sediments northeast of Svalbard: Mn(IV) and Fe(III) reduction as principal anaerobic respiratory pathways. Mar. Ecol. Prog. Ser. 322, 15–27. ( 10.3354/meps322015) [DOI] [Google Scholar]
- 50.Pathirana I, Knies J, Felix M, Mann U. 2014. Towards an improved organic carbon budget for the western Barents Sea shelf. Clim. Past 10, 569–587. ( 10.5194/cp-10-569-2014) [DOI] [Google Scholar]
- 51.Tamelander T, Reigstad M, Hop H, Carroll ML, Wassmann P. 2008. Pelagic and sympagic contribution of organic matter to zooplankton and vertical export in the Barents Sea marginal ice zone. Deep Sea Res. Part II Top. Stud. Oceanogr. 55, 2330–2339. ( 10.1016/j.dsr2.2008.05.019) [DOI] [Google Scholar]
- 52.Zaborska A, Carroll J, Papucci C, Torricelli L, Carroll ML, Walkusz-Miotk J, Pempkowiak J. 2008. Recent sediment accumulation rates for the Western margin of the Barents Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 55, 2352–2360. ( 10.1016/j.dsr2.2008.05.026) [DOI] [Google Scholar]
- 53.Morata N, Renaud PE. 2008. Sedimentary pigments in the western Barents Sea: a reflection of pelagic–benthic coupling? Deep Sea Res. Part II Top. Stud. Oceanogr. 55, 2381–2389. ( 10.1016/j.dsr2.2008.05.004) [DOI] [Google Scholar]
- 54.Belt ST, Cabedo-Sanz P, Smik L, Navarro-Rodriguez A, Berben SMP, Knies J, Husum K. 2015. Identification of paleo Arctic winter sea ice limits and the marginal ice zone: optimised biomarker-based reconstructions of late Quaternary Arctic sea ice. Earth Planet. Sci. Lett. 431, 127–139. ( 10.1016/j.epsl.2015.09.020) [DOI] [Google Scholar]
- 55.Knies J, Martinez P. 2009. Organic matter sedimentation in the western Barents Sea region: terrestrial and marine contribution based on isotopic composition and organic nitrogen content. Nor. J. Geol. 89, 79–89. [Google Scholar]
- 56.Knies J, Jensen HKB, Finne TE, Lepland A, Sæther OM. 2006. Sediment composition and heavy metal distribution in Barents Sea surface samples: Results from Institute of Marine Research 2003 and 2004 cruises. Geological Survey of Norway; 2006. Report No.: 2006.067.
- 57.Maiti K, Carroll J, Benitez-Nelson CR. 2010. Sedimentation and particle dynamics in the seasonal ice zone of the Barents Sea. J. Mar. Syst. 79, 185–198. ( 10.1016/j.jmarsys.2009.09.001) [DOI] [Google Scholar]
- 58.Hulth S, Blackburn TH, Hall POJ. 1994. Arctic sediments (Svalbard): consumption and microdistribution of oxygen. Mar. Chem. 46, 293–316. ( 10.1016/0304-4203(94)90084-1) [DOI] [Google Scholar]
- 59.Stein R, Grobe H, Wahsner M. 1994. Organic carbon, carbonate, and clay mineral distributions in eastern central Arctic Ocean surface sediments. Mar. Geol. 119, 269–285. ( 10.1016/0025-3227(94)90185-6) [DOI] [Google Scholar]
- 60.Steinsund PI, Hald M. 1994. Recent calcium carbonate dissolution in the Barents Sea: paleoceanographic applications. Mar. Geol. 117, 303–316. ( 10.1016/0025-3227(94)90022-1) [DOI] [Google Scholar]
- 61.Knies J, et al. 2016. Sea-ice dynamics in an Arctic coastal polynya during the past 6500 years. Arktos 3, 1–15. [Google Scholar]
- 62.Schauer U. 1995. The release of brine-enriched shelf water from Storfjord into the Norwegian Sea. J. Geophys. Res. 100, 16 015–16 028. ( 10.1029/95JC01184) [DOI] [Google Scholar]
- 63.Honjo S, Manganini SJ, Wefer G. 1988. Annual particle flux and a winter outburst of sedimentation in the northern Norwegian Sea. Deep Sea Res. A 35, 1223–1234. ( 10.1016/0198-0149(88)90078-7) [DOI] [Google Scholar]
- 64.Hebbeln D, Henrich R, Baumann KH. 1998. Paleoceanography of the last interglacial/glacial cycle in the Polar North Atlantic. Quat. Sci. Rev. 17, 125–153. ( 10.1016/S0277-3791(97)00067-X) [DOI] [Google Scholar]
- 65.Berben SMP, Husum K, Cabedo-Sanz P, Belt ST. 2014. Holocene sub-centennial evolution of Atlantic water inflow and sea ice distribution in the western Barents Sea. Clim. Past 10, 181–198. ( 10.5194/cp-10-181-2014) [DOI] [Google Scholar]
- 66.Wassmann P, Peinert R, Smetacek V. 1991. Patterns of production and sedimentation in the boreal and polar Northeast Atlantic. Polar Res. 10, 209–228. ( 10.1111/j.1751-8369.1991.tb00647.x) [DOI] [Google Scholar]
- 67.Boetius A, et al. 2013. Export of algal biomass from the melting Arctic sea ice. Science 339, 1430–1432. ( 10.1126/science.1231346) [DOI] [PubMed] [Google Scholar]
- 68.Reigstad M, Wexels Riser C, Wassmann P, Ratkova T. 2008. Vertical export of particulate organic carbon: attenuation, composition and loss rates in the northern Barents Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 55, 2308–2319. ( 10.1016/j.dsr2.2008.05.007) [DOI] [Google Scholar]
- 69.Rasmussen TL, Thomsen E. 2013. Pink marine sediments reveal rapid ice melt and Arctic meltwater discharge during Dansgaard–Oeschger warmings. Nat. Commun. 4, 2849 ( 10.1038/ncomms3849) [DOI] [PubMed] [Google Scholar]
- 70.Flink AE, Noormets R, Fransner O, Hogan KA, ÓRegan M, Jakobsson M. 2017. Past ice flow in Wahlenbergfjorden and its implications for late Quaternary ice sheet dynamics in northeastern Svalbard. Quat. Sci. Rev. 163, 162–179. ( 10.1016/j.quascirev.2017.03.021) [DOI] [Google Scholar]
- 71.Vogt C, Knies J. 2009. Sediment pathways in the western Barents Sea inferred from clay mineral assemblages in surface sediments. Nor. J. Geol. 89, 41–55. [Google Scholar]
- 72.Lannuzel D, et al. 2016. Iron in sea ice: review and new insights. Elem. Sci. Anth. 4, 000130. [Google Scholar]
- 73.Elverhoi A, Pfirman SL, Solheim A, Larssen BB. 1989. Glaciomarine sedimentation in epicontinental seas exemplified by the northern Barents Sea. Mar. Geol. 85, 225–250. ( 10.1016/0025-3227(89)90155-2) [DOI] [Google Scholar]
- 74.Bjørlykke K, Bue B, Elverhøi A. 1978. Quaternary sediments in the northwestern part of the Barents Sea and their relation to the underlying Mesozoic bedrock. Sedimentology 25, 227–246. ( 10.1111/j.1365-3091.1978.tb00310.x) [DOI] [Google Scholar]
- 75.Chen C, Dynes JJ, Wang J, Sparks DL. 2014. Properties of Fe-organic matter associations via coprecipitation versus adsorption. Environ. Sci. Technol. 48, 13 751–13 759. ( 10.1021/es503669u) [DOI] [PubMed] [Google Scholar]
- 76.Wagai R, Mayer LM. 2007. Sorptive stabilization of organic matter in soils by hydrous iron oxides. Geochim. Cosmochim. Acta 71, 25–35. ( 10.1016/j.gca.2006.08.047) [DOI] [Google Scholar]
- 77.Stevenson MA, Abbott GD. 2019. Exploring the composition of macromolecular organic matter in Arctic Ocean sediments under a changing sea ice gradient. J. Anal. Appl. Pyrolysis 140, 102–111. ( 10.1016/j.jaap.2019.02.006) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.