Abstract
The iconic picture of Arctic marine ecosystems shows an intense pulse of biological productivity around the spring bloom that is sustained while fresh organic matter (OM) is available, after which ecosystem activity declines to basal levels in autumn and winter. We investigated seasonality in benthic biogeochemical cycling at three stations in a high Arctic fjord that has recently lost much of its seasonal ice-cover. Unlike observations from other Arctic locations, we find little seasonality in sediment community respiration and bioturbation rates, although different sediment reworking modes varied through the year. Nutrient fluxes did vary, suggesting that, although OM was processed at similar rates, seasonality in its quality led to spring/summer peaks in inorganic nitrogen and silicate fluxes. These patterns correspond to published information on seasonality in vertical flux at the stations. Largely ice-free Kongsfjorden has a considerable detrital pool in soft sediments which sustain benthic communities over the year. Sources of this include macroalgae and terrestrial runoff. Climate change leading to less ice cover, higher light availability and expanded benthic habitat may lead to more detrital carbon in the system, dampening the quantitative importance of seasonal pulses of phytodetritus to seafloor communities in some areas of the Arctic.
This article is part of the theme issue ‘The changing Arctic Ocean: consequences for biological communities, biogeochemical processes and ecosystem functioning'.
Keywords: bioturbation, Kongsfjorden, nutrient fluxes, phytodetritus, respiration, Svalbard
1. Introduction
One of the dominant concepts guiding current understanding of the functioning of Arctic marine ecosystems is the overriding role of dramatic seasonality in physical drivers. Indeed, intense but short-lived algal blooms characterize many Arctic areas and are coupled to the return of sunlight, melting of sea ice and stratification of the water column. These blooms fuel both pelagic and benthic food-webs, and sustain migratory fish, seabirds and mammals during the short summer. The lack of primary production during the long polar night has resulted in an assumption of dormancy in many ecosystem elements [1], but this has recently been challenged by evidence of active functioning throughout the ecosystem [2,3], including by seafloor communities [4].
For Arctic seafloor communities, generally presumed to be limited by labile carbon [5], ecological functioning shows strong spatial [6,7] and seasonal [8–12] variability linked to patterns of food availability. In spring and summer, intense pulses of fresh material can reach the seafloor, such as ice-derived material or phytodetritus [13,14], upon which benthic communities react in both field and experimental studies by quickly increasing respiration and bioturbation rates [11,15]. These findings are consistent with the paradigm of pelagic–benthic coupling, which posits that stocks and processes in seafloor communities generally mirror spatial and temporal patterns in pelagic production and flux [16,17].
Such coupling has been suggested to be more pronounced in high-latitude environments due in large part to the pulsed or spatially discrete nature of food arrival on the seafloor [18]. Many of the pioneering studies of these processes, however, have been conducted on open shelves and areas with considerable sea-ice cover. Climatic change has led to strong declines in sea-ice cover, thickness and the length of the sea-ice season over the past decades [19], and has resulted in changes in phenology and composition of phytoplankton blooms and flux patterns [20,21]. Furthermore, increased temperature and light availability (due to reduced ice cover) result in increased benthic algal production in near shore regions [22], with predictions of larger inputs of (macro-) algal detritus to benthic systems in a warmer Arctic. How predicted and observed changes in amount and seasonality of labile carbon will affect Arctic seafloor communities is unclear, but their processes are vital to regenerating nutrients in these otherwise oligo- to meso- trophic systems. Furthermore, fjords are sites of disproportionately high carbon sequestration on a global scale [23], and climate-driven changes in cycling may have implications for climate feedback processes.
Kongsfjorden, a high Arctic fjord on the west coast of Svalbard, has observed a dramatic reduction in seasonal ice-cover and, except for inner bays, has been largely ice-free in winter since 2006 [24]. The fjord still exhibits strong seasonality in primary production and vertical fluxes to the seafloor [25,26] but little seasonal variability in infaunal community structure [27,28]. Sediment processes, such as oxygen and nutrient fluxes and bioturbation rates, have not been investigated but are critical to understanding ecosystem functioning in rapidly changing Arctic environments.
In this study, we quantify seasonal differences in (1) sediment properties, (2) bioturbation and biogeochemical-cycling rates at three locations in Kongsfjorden with different ice-cover and infauna-community composition and (3) identify mechanisms linking environmental drivers and ecological processes. Results offer insight into the response of benthic communities to a changing Arctic, and how current paradigms of ecosystem functioning may be challenged over the coming decades.
2. Material and methods
(a). Study area
Kongsfjorden (79° N and 12° E) is 27 km long, 10 km wide and is located on the Northwestern part of Spitsbergen Island in the Svalbard Archipelago (figure 1). Because of its open connection to Fram Strait, it is largely influenced by advection of transformed Atlantic waters [29]. In the past, the fjord was regularly covered by sea ice, but because of increased advection of Atlantic water, it experiences variable and reduced sea-ice, with mostly ice-free years since 2005/2006 [29]. The inner part of the fjord is still rather ‘Arctic' because of the influence of four large tidal glaciers.
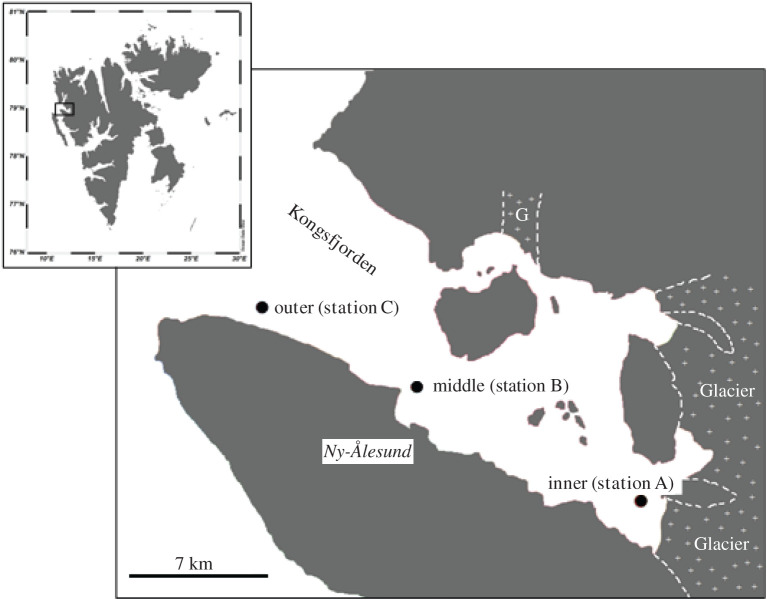
Figure 1.
Locations of the three stations at inner (station A), middle (station B), outer (station C) in Kongsfjorden (Svalbard).
Three sampling stations were selected along a gradient from the inner part of the fjord, to the fjord opening: the inner-fjord station (78°54 N, 12°28 E, 80 m deep, station A) was located at 1.4 km from the head of the fjord under the direct influence of the tidal glacier inputs; the middle-fjord station (78°567 N, 11°56E, 295 m deep, station B) was located midway between the tidal glacier and the ocean; and the outer station (78°59 N, 11°32 E, 305 m deep, station C) was located 24 km from the head of the fjord, near the fjord mouth. All stations were visited in May, August and October 2012, and in August 2014 and January 2015. However, due to technical difficulties, it was not possible to perform sediment cores incubations in May and August 2012 at the inner station. The middle and outer stations were also visited in January 2013 period.
(b). Field sampling
During each field campaign, nine sediment cores (12.7 cm diameter, 10 cm height) were collected from each station with a HAPS corer (KC Denmark), except in January 2013 where the sediment cores were collected using a box corer (45 × 45 cm). The top 2 cm of sediment from three cores were sliced after collection for determination of the sedimentary organic matter (OM) quantity and quality (e.g. chlorophyll and phaeopigments; total organic carbon and total nitrogen contents: TOC, TN). Sediments were gently homogenized and frozen (−20°C) for further analyses. The six remaining cores were kept in a dark cold room at 2°C for benthic processes measurements.
(c). Sediment core incubation for benthic fluxes and bioturbation activities measurements
Sediment cores were filled (approx. 1 l) with recently collected bottom water (2–3°C, 35‰, [30]) and aerated to keep oxygen saturation. The cores were acclimated in the cold room for 24 h (2–3°C), whereupon overlying water was changed to remove the released metabolites, and 5 g of fluorescent luminophores (red, 63–90 µm) were homogeneously introduced to the overlying water to quantify biological sediment reworking activity [7]. In order to measure nutrient fluxes and sediment oxygen demand (SOD), cores were then sealed using tops that provided constant stirring of the overlying water [15]. Oxygen concentrations were monitored every 4–6 h using spot sensors (Presens A/S; Germany) [15]. Incubations were terminated after 24 h when 15–20% of the oxygen had been consumed and SOD was measured as the (negative) slope of the regression line between oxygen concentration and time [15].
Sixty millilitres of overlying waters were sampled with a syringe at the beginning and at the end of the 24 h incubation for nutrient analyses. Nutrient fluxes were measured as a difference in nutrient concentration between the two sampling times [15]. Water samples for nitrate + nitrite ( + ), ammonium () and phosphate () were filtered (0.2 µm) and directly frozen for future analyses, while filtered samples for silicate ( ) were kept at +4°C. Cores were then reopened and kept with constant aeration by bubbling and by renewing overlying water every 3 days. Cores were incubated in those conditions with the luminophores for 10 days [7]. At the end of the experiment, the sediment cores were sliced into 0.5 cm layers from 0 to 5 cm depth, 1-cm layers from 5 to 10 cm depth and 2 cm layers from 10 to 18 cm depth. Each slice was carefully homogenized and kept for luminophores quantification.
(d). Laboratory analyses
(i). Bulk sediment analyses
At the laboratory, sediments were freeze-dried and gently crushed to powder and homogenized for bulk sediment analyses. Dry bulk sediment density (δ) was determined for each sample by using the known sediment volume. Pigments (chlorophyll a (chla) and phaeopigments (phaeo)) were analysed fluorometrically [15]. OM content was measured as loss on ignition (450°C, 4 h). TOC and TN contents were measured by the Thermo Quest Flash EA 1112 CHN analyser [15]. Biomass of TOC and pigments contents was calculated (TOCbm, chlabm and phaeobm) as ratios (chla/phaeo; C-chla/TOC) over the 0–2 cm sediment layer.
(ii). Nutrient fluxes
Nitrate + nitrite, silicate and phosphate were analysed by colorimetry on a Bran + Luebbe Autoanalyzer 3, and ammonium was analysed by fluorometry [15]. Analytical precision for nutrient analyses was 0.05 µM. Fluxes were calculated from the slopes of the linear regressions of nutrient concentrations against time.
(iii). Bioturbation measurements
Luminophore detection and counting for quantification of the tracer transport modes were made following [7]. This model simultaneous quantifies the biodiffusion-like transport (Db coefficient; cm2 . y−1) and the non-local transport of the tracers (r coefficient; y−1). It adjusts a theoretical curve of the tracer distribution with depth on experimental data. The best fit between the observed and modelled tracer distribution is estimated by the least-squares method and produces the best Db and r coefficients.
(e). Data analyses
The influences of time and location on biogeochemical fluxes, sediment variables and bioturbation coefficients were tested using a two-way analysis of variance with season (January, May, August, October from 2012 to 2015) and stations (inner, middle and outer) as fixed factors. To satisfy homoscedasticity and normality requirements, oxygen and silicate fluxes were log(x + 1) and square-root transformed, respectively. Parametric analyses (ANOVA) and pairwise comparison tests (Tukey's HSD) were then applied to these variables and to TOC and TN. Differences in other parameters (C/N, TOCbm, chlabm, phaeobm, C-chla/TOC, chla/phaeo, ammonia, nitrate + nitrite and phosphate fluxes, Db and r coefficients) were tested using non-parametric two-way ANOVA (Sheirer–Ray–Hare test) followed by the Wilcoxon pairwise comparison test with Bonferroni corrections, due to the non-homoscedasticity of the data and the unbalanced design [31].
In order to determine which biogeochemical variables, benthic fluxes and bioturbation coefficients explained most variability among studied stations between 2012 and 2015, a principle components analysis (PCA) was performed using the ‘factomine' Rstudio package [32]. Data were normalized prior to analysis.
Correlation analyses were then used to quantify the relatedness between variables using the non-parametric Spearman coefficient after data normality verification.
3. Results
(a). Sediment organic matter and pigments
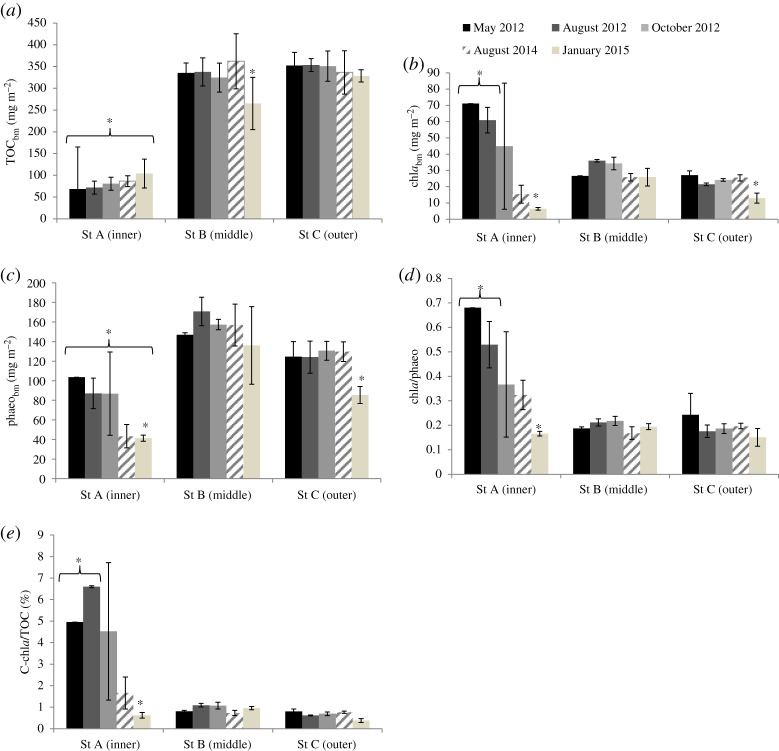
OM (9%), TOC (1.6%) and TN (0.2%) were significantly higher at the outer station ‘C', followed by the middle station ‘B' and the inner station ‘A' (table 1, electronic supplementary material, figure S1). No temporal changes in % OM, TOC and TN were observed at station A. In the outer station, OM, TOC and TN contents were significantly higher in January 2015 than in August 2014 and October 2012 (table 1; electronic supplementary material, figure S1). OM followed the same trends in the middle station, but TOC was lower there in January 2015 than in August 2012, 2014 and May 2012 (table 1; electronic supplementary material, figure S1). Significantly lower biomass of TOC (TOCbm ∼ 50–100 mg m−2) was found at station A compared to the two other stations for each date (table 1 and figure 2a; electronic supplementary material, figure S1). Seasonal decreasing of TOCbm was only statistically significant at station B in January 2015 (figure 2a). Biomass of chla (chlabm) was highest (60–70 mg m−2)) near the glacier in May and August 2012 (table 1 and figure 2b). While the chlabm remained stable over time at station B, it significantly decreased in January 2015 in stations A and C (10 mg m−2; figure 2b and table 1). Biomass of phaeopigments (phaeobm) was significantly different between all station pairs, being significantly the lowest near the glacier (50 mg m−2), followed by the outer (approx. 120 mg m−2) and middle (approx. 150 mg m−2) stations (figure 2c). phaeobm did not change seasonally at station B, but significantly decreased in January 2015 at the two other stations (table 1 and figure 2c). chla:phaeo and C-chla:TOC ratios were both significantly higher at the station near the glacier in May and August 2012, whereas it decreased significantly in January 2015. chla:phaeo ratios and C-chla:TOC ratios were consistent at the middle and outer stations for the entire sampling period. Pigment biomass and their respective ratios were highly variable in October between the sediment cores at station A (table 1 and figure 2).
Table 1.
Differences in the bulk sediment variables, benthic fluxes and bioturbation coefficients at the three stations of Svalbard (inner (A), middle (B), outer (C)) between 2012 (May, August and October), 2014 (August) and 2015 (January), after two-way ANOVAs (A; F and p-values) or Sheirer–Ray–Hare (SRH; H and p-values) tests. Significant differences (p < 0.05) are indicated by an asterisk (*). Last column indicates the pair wise comparisons between the three stations.
| main effect |
main effect |
interaction |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| time |
station |
time × station |
pairwise comparison between stations |
|||||||
| test | stat | p | stat | p | stat | p | inner station | middle station | outer station | |
| OM (%) | A | 5.7 | 0.004** | 26.6 | 0.00001*** | 5.4 | 0.001** | a | b | c |
| TOC (%) | A | 12.7 | 0.00001*** | 849 | 0.00001*** | 7.3 | 0.00001*** | a | b | c |
| TON (%) | A | 12 | 0.00001*** | 1112 | 0.00001*** | 4.8 | 0.001** | a | b | c |
| C:N (mol:mol) | SRH | 30 | 0.00001*** | 36 | 0.00001*** | 39 | 0.00001*** | a | a | a |
| TOCbm (mg m-2) | SRH | 14 | 0.006** | 70 | 0.00001*** | 15 | 0.04* | a | b | b |
| chlabm (mg m-2) | SRH | 24 | 0.00001*** | 24 | 0.00001*** | 38 | 0.00001*** | a | b | b |
| phaeobm (mg m-2) | SRH | 6.7 | 0.10 | 54 | 0.00001*** | 17 | 0.03* | a | b | c |
| C-chla:TOC | SRH | 7.6 | 0.1 | 70.4 | 0.00001*** | 31.6 | 0.0001** | a | b | b |
| chla:phaeo | SRH | 6.5 | 0.16 | 36.5 | 0.00001*** | 35 | 0.00001*** | a | b | b |
| O2 (mmol m-2 d-1) | A | 2 | 0.12 | 6.5 | 0.002** | 2.8 | a | b | b | |
| (mmol m-2 d-1) | SRH | 47 | 0.001** | 88 | 0.0001** | 53 | 0.01* | a | a | b |
| (mmol m-2 d-1) | SRH | 69 | 0.01* | 12 | 52 | a | a | a | ||
| (mmol m-2 d-1) | A | 30 | 0.001** | 28 | 0.0001** | 4.5 | 0.0001** | a | b | c |
| (mmol m-2 d-1) | SRH | 62 | 0.001** | 41 | 0.01* | 112 | 0.01* | a | b | a |
| Db (cm2 y-1) | SRH | 68 | 0.001** | 11 | 0.004** | 7 | a | b | b | |
| R (y−1) | SRH | 65 | 0.001** | 74 | 0.001** | 12 | a | b | b | |
Figure 2.
Mean biomass (mg m−2) of total organic carbon (TOC) (a), chla (b), phaeopigments (c) and mean ratios of chla:phaeo (d) and C-chla:TOC (%) (e) over the 2 first cm of sediment (±s.d.) at the three stations in Kongsfjorden, Svalbard (inner (A), middle (B), outer (C)) in 2012 (May, August and October), 2014 (August) and 2015 (January) (mean ± s.d., n = 3). * indicate biomass or ratios that significantly differ with station and with time (p < 0.05).
(b). Bioturbation coefficients
The biodiffusion coefficient Db ranged from 0 to 3 cm2 y−1 along the fjord; mean values were significantly higher in May 2012 (2–3 cm2 y−1), than in August 2012, 2014, October 2012 and January 2015 (Db < 0.5 cm2 y−1; p < 0.00002) for the middle and outer stations (table 1 and figure 3). The non-local transport coefficient (r) ranged from 0 to 8 y−1 over the study area and was significantly lowest at the inner station in October 2012, August 2014 and January 2015 (table 1 and figure 3). While the mean r remained stable over time at the middle station, it was significantly higher in October 2012 for the outer station (p < 0.01) (table 1 and figure 3).
Figure 3.
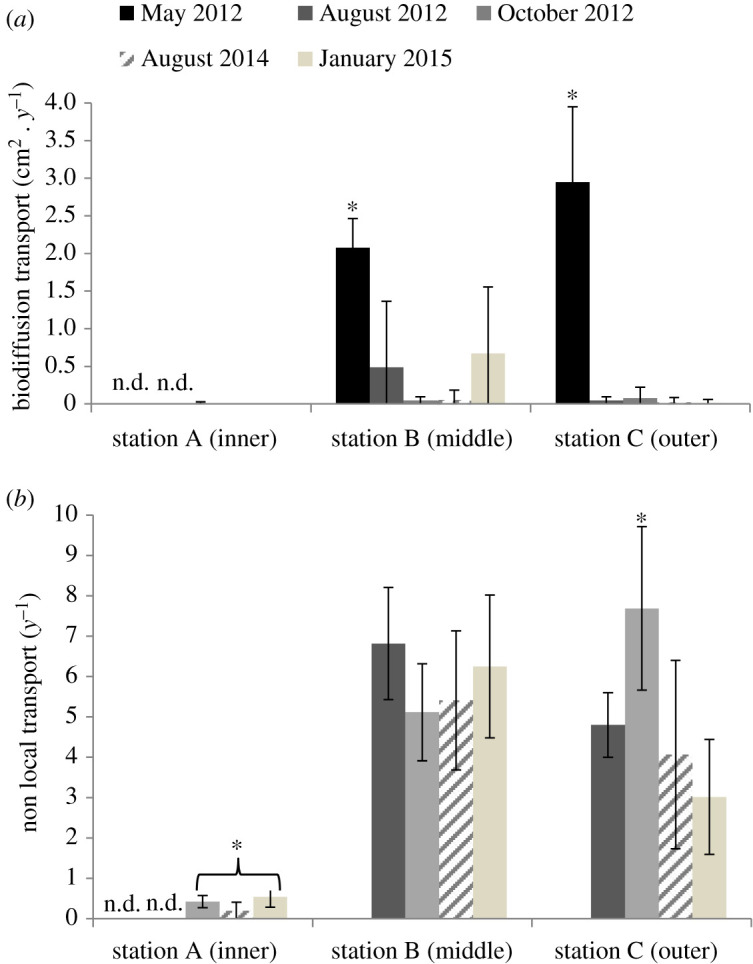
Mean biodiffusion (Db in cm2 y−1) and non-local (r in y−1) transports measured at the three stations in Kongsfjorden (inner (A), middle (B), outer (C)) between 2012 and 2015 (mean ± s.d., n = 6). * indicates bioturbation coefficients that significantly differ with station and with time (p < 0.05). The station A presented no data (n.d.) in May and August 2012 since sediment cores incubations were not possible at this period. The absence of bars in August 2014 and January 2015 at station A and in May 2012 at stations B and C means that no bioturbation was measured.
(c). Oxygen and nutrient fluxes
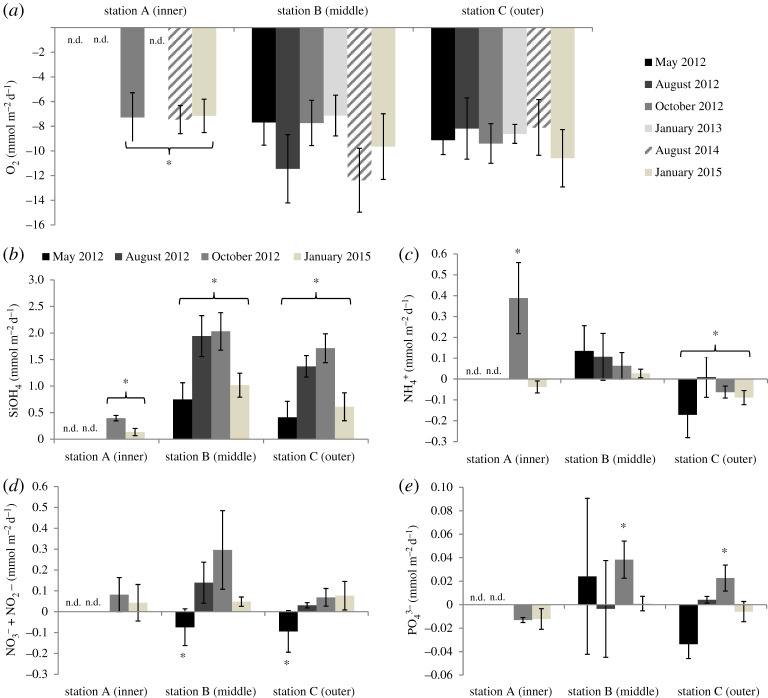
Station A presented no data (n.d.) in May, August 2012 and January 2013 since core incubation at this station was not possible during these months. SOD was lowest at the inner station in October 2012, August 2014 and in January 2015 (figure 4a and table 1) and remained stable over the three sampling periods (6–7 mmol O2 m−2 d−1). For the middle and outer stations, SOD was similar (10–12 mmol m−2 d−1) and did not vary seasonally (p > 0.12, table 1), although SOD tended to be higher in the months of August 2012 and 2014 at the middle station. Nutrient fluxes were measured at the middle and outer stations in May, August, October 2012 and January 2015 and during October 2012 and January 2015 at the inner station (figure 4b–e and table 1). Silicate was always released from the sediment and significant highest rates were at the middle and outer stations (1.5–2 mmol m−2 d−1, p < 0.05), mainly in August and October 2012 (figure 4b and table 1). Ammonium uptake was significant and consistent at the outer station (−0.1 mmol m−2 d−1; p < 0.05, table 1 and figure 4c). Low levels of ammonium release were measured in the middle station (0.1 mmol m−2 d−1) with no significant seasonal change. At station A, ammonia fluxes were opposite with high ammonia release in October 2012 (0.4 mmol m−2 d−1) and ammonia uptake in January 2015 (−0.5 mmol m−2 d−1). Nitrate + nitrite showed a consistent pattern going from significant NO3 + NO2 uptake in May 2012 (p < 0.05, table 1) at stations B and C to release in October, August 2012 and January 2015 for all stations (figure 4d). Net phosphate fluxes were quite low (<0.04 mmol m−2 d−1) and showed different significant seasonal patterns among the three stations (figure 4e and table 1, p < 0.05). Phosphate influxes were measured at station A in October 2012 and in January, and in station C in May and January (figure 4d). Phosphate was released and significantly highest in October 2012 at the stations B and C (p < 0.05, table 1).
Figure 4.
Mean oxygen (a) and nutrient net fluxes (b–e) (mmol m−2 d−1) at the sediment–water interfaces at the three stations in Kongsfjorden (inner (A), middle (B), outer (C)) between 2012 and 2015 (mean ± s.d., n = 6). * indicates biogeochemical fluxes that significantly differ with station and with time (p < 0.05). The station A presented no data (n.d.) when core sampling was not feasible.
(d). Environmental variables
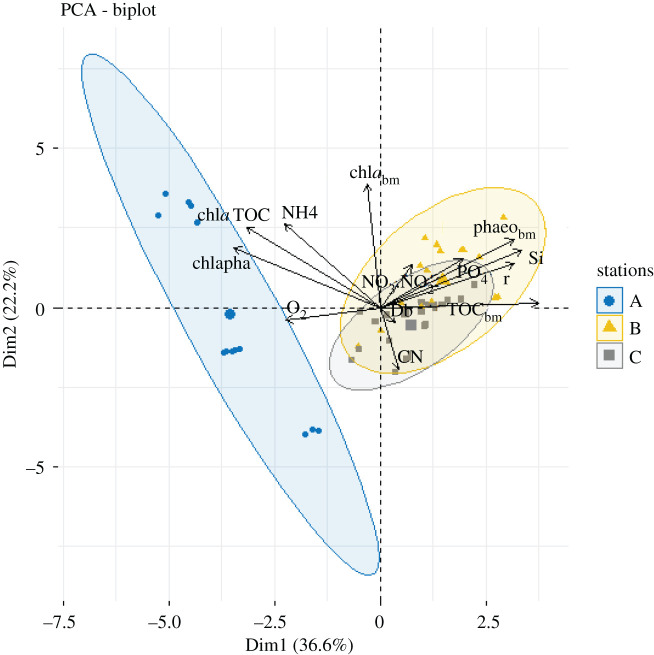
The PCA analysis explained 59% of the variability among sampling stations and dates: the first axis explained 37% and the second axis 22% (figure 5; electronic supplementary material, figure S2). The most important variables (and their loadings) defining the first axis were TOCbm (0.89), silicate fluxes (0.80), r (0.76), phaeobm (0.75), fluxes (0.46), SOD (−0.54), fluxes (−0.54), C-chla:TOC (−0.75), chla:phaeo (−0.83), while chlabm (0.96), chla (0.93), fluxes (0.63), C-chla:TOC (0.60), phaeobm (0.51) and C:N (−0.46) defined the second axis. Stations were separated into two groups on the basis of PCA results: station A alone and stations B and C together. Time points at station A are distributed along a gradient from lowest values of chla and flux in January to highest in October. The opposite gradient was observed for C/N. Sampling date for stations B and C had little effect on station groupings, and these stations were characterized by the PCA as having high SOD, phaeobm, TOCbm, nitrate + nitrite, phosphate and silicate fluxes, and non-local bioturbation rates. Biogeochemical fluxes were significantly inter-correlated, SOD was positively correlated with ammonium uptake, and silicate and phosphate efflux to overlying waters as well as chla:phaeo and C-chla:TOC ratios (table 2). SOD was negatively correlated with phaeobm and non-local bioturbation (table 2). chlabm was positively correlated with ammonium flux (table 2). Both biodiffusion and nitrate + nitrite fluxes were not correlated with any measured variables.
Figure 5.
Principal component analysis based on the sediment variables (C:N, chla:phaeo and C-chla:TOC ratios, biomass of TOC, chla and phaeo pigments), benthic fluxes (oxygen, ammonium (NH4), nitrate + nitrite (NO3 + NO2), phosphate (), silicate , and bioturbation coefficients (biodiffusion, Db and bioadvection, r) along the Kongsfjorden area between 2012 and 2015. The two axes pictured explain 58.8% of the variance in station data. (Online version in version.)
Table 2.
Spearman's rank correlation analyses between biogeochemical fluxes (O2, + , , ), sediment biomarkers (C:N, C-chla:TOC, chla:phaeo ratios, and biomass of TOC, chla and phaeopigments), and bioturbation variables (Db and r). Significant values are marked in bold (p < 0.05).
| Db | r | O2 | + | Si(OH)4 | C:N | chla:phaeo | C-chla:TOC | TOCbm | chlabm | phaeobm | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Db | 1.00 | −0.20 | −0.12 | −0.27 | −0.08 | −0.43 | −0.29 | −0.18 | −0.04 | −0.16 | 0.34 | −0.07 | 0.19 |
| r | −0.20 | 1.00 | −0.86 | 0.49 | 0.94 | −0.42 | 0.80 | 0.06 | −0.72 | −0.69 | 0.77 | −0.30 | 0.76 |
| O2 | −0.12 | −0.86 | 1.00 | −0.38 | −0.94 | 0.59 | −0.65 | 0.00 | 0.71 | 0.72 | −0.92 | 0.24 | −0.91 |
| + | −0.27 | 0.49 | −0.38 | 1.00 | 0.45 | 0.08 | 0.15 | −0.53 | 0.08 | 0.08 | 0.17 | 0.36 | 0.41 |
| Si(OH)4 | −0.08 | 0.94 | −0.94 | 0.45 | 1.00 | −0.47 | 0.80 | 0.01 | −0.72 | −0.71 | 0.83 | −0.21 | 0.87 |
| −0.43 | −0.42 | 0.59 | 0.08 | −0.47 | 1.00 | −0.10 | −0.37 | 0.82 | 0.86 | −0.73 | 0.79 | −0.36 | |
| −0.29 | 0.80 | −0.65 | 0.15 | 0.80 | −0.10 | 1.00 | 0.18 | −0.59 | −0.51 | 0.59 | −0.16 | 0.66 | |
| C:N | −0.18 | 0.06 | 0.00 | −0.53 | 0.01 | −0.37 | 0.18 | 1.00 | −0.48 | −0.44 | 0.12 | −0.71 | −0.26 |
| chla:phaeo | −0.04 | −0.72 | 0.71 | 0.08 | −0.72 | 0.82 | −0.59 | −0.48 | 1.00 | 0.98 | −0.79 | 0.81 | −0.50 |
| C‐chla:TOC | −0.16 | −0.69 | 0.72 | 0.08 | −0.71 | 0.86 | −0.51 | −0.44 | 0.98 | 1.00 | −0.82 | 0.80 | −0.53 |
| TOCbm | 0.34 | 0.77 | −0.92 | 0.17 | 0.83 | −0.73 | 0.59 | 0.12 | −0.79 | −0.82 | 1.00 | −0.43 | 0.86 |
| chlabm | −0.07 | −0.30 | 0.24 | 0.36 | −0.21 | 0.79 | −0.16 | −0.71 | 0.81 | 0.80 | −0.43 | 1.00 | 0.04 |
| phaeobm | 0.19 | 0.76 | −0.91 | 0.41 | 0.87 | −0.36 | 0.66 | −0.26 | −0.50 | −0.53 | 0.86 | 0.04 | 1.00 |
4. Discussion
(a). Spatial and temporal differences in sediment properties
In Kongsfjorden, previous studies have shown seasonal changes in pelagic production. In 2012, a mooring in mid-Kongsfjorden registered a strong diatom-dominated spring bloom from April to mid-May, with moderate fluorescence from mid-June until July [26]. A seasonal sediment-trap study conducted from 2012 to 2013 documented a sedimenting bloom in May as increased particulate organic carbon (POC) and chla flux at all three stations, continuing into August at the glacier station (station A) [25]. Another study [33] found that the sediments near the glacier were lithogenic-dominant depleted in biopolymeric carbon (the sum of the main macromolecules: lipids, proteins and carbohydrates) compared to the entrance of the fjord and that the sedimentary isotopic signature showed different sources between the inner (fresh diatoms, lithogenic material with calcareous benthic foraminifers) and outer (faecal pellets, degraded phytoplankton cells) stations. Recently, non-pelagic sources of organic carbon have been suggested to be important for biogeochemical cycling in Kongsfjorden and many coastal sites in the Arctic. These other sources, including macroalgal detritus [11], terrestrially derived carbon [34,35] and microphytobenthos [36], may be partly responsible for the higher sedimentary TOC at middle and outer Kongsfjorden stations, whereas the high inorganic sediment load at the glacier station dilutes any of these inputs resulting in consistently low TOC levels. These sources were not measured in this study, however.
It has been suggested that changes in the quality of the OM on the seafloor can be reflected in sedimentary TOC or pigments [37]. Although sediment OM, TC, TN contents and TOCbm were higher in the middle and outer stations in our study, biogenic silica and fecal-pellet carbon fluxes, indicating zooplankton grazing of diatoms at these same stations in May [25], likely explain the low chla/phaeo and C-chl/TOC ratios in sediments at stations B and C year-round. Low zooplankton grazing is suggested for station A [25,33], allowing fresher sedimenting material at this station.
Although temporal changes were not reflected in sediment organic parameters (OM, TC, TN, TOCbm) at station A (glacier station), temporal decline in sediment pigments (chla, phaeopigments, chla/phaeo and C-chl/TOC ratios) were observed at this station. This is perhaps to be expected due to the seasonal sources of phytopigments relative to the larger pool of OC of mixed lability in the sediments. This shows that sediment pigment content and biomass are determined by the intensity of POC (grazed, faecal pellets) and phytoplankton (ungrazed) downward export fluxes over the year. Elevated export fluxes observed in May 2012 near the station result from the phytoplankton bloom and the release of particles from glacier melting, while during the important melt period in August the turbid waters likely dampen phytoplankton development [25]. This is more obvious in August 2014, which was a warm year for the Kongsfjorden with the inner section receiving warm Atlantic waters accelerating the glacier melt [38–40]. The month of January in this region is marked by a decline in the development of autotrophic organisms and in glacial melt both caused by the colder temperatures and polar night [25]. Once deposited at the sediment surface, fresh organic material (ungrazed) is slowly consumed over the following months since the benthic biomass and diversity [27], and bioturbation activities (this study) remain low at this station. By contrast, the export fluxes observed in the middle and outer stations remained elevated and stable over time due to the continuous intrusion of warm Atlantic waters and associated phytoplankton communities until the middle of the fjord [25,39], favouring the match between phytoplankton bloom and copepod grazing, and POM fluxes. However, sedimenting OM does not accumulate at stations B and C, suggesting it is consumed and processed rapidly by seafloor communities. These two stations have higher faunal abundance, species and functional diversity than station A [27]. Higher bioturbation rates at these stations than at station A also suggest that benthic communities at these stations are more efficient at consuming material upon its arrival on the sediment surface. A similar finding was made in a study on the Beaufort shelf, where, despite strong seasonal signals in vertical flux and sediment community oxygen consumption, little change in sediment pigments was found [11].
(b). Spatial and temporal differences in bioturbation activities
Bioturbation showed strong spatial variability in the fjord, with very low biodiffusion and non-local mixing at station A. This station has no deep burrowing polychaetes (e.g. maldanids) and is dominated by small surface-deposit feeders (cirratulid and cossurid polychaetes) throughout the year [27]. Non-local mixing rates at station A were similar to those for the deep stations (over 400 m) Barents Sea region dominated by small organisms [7]. The middle and outer stations showed similar rates of both biodiffusion and non-local mixing, and these rates were 2–4× those from previous studies in Arctic [7]. Active burrowers and deep-dwelling tubeworms were found at stations B and C [27], and organic content was 3–4 × higher at the middle and outer stations than at station A (figure 3).
There was little obvious seasonal change in non-local transport at any of the stations, and non-local transports are positively correlated to degraded sedimentary OM (phaeopigment biomass). This is consistent with the lack of accumulation of a pulse of phytopigments at the mid and outer stations (figure 3), and the lower richness and abundance of burrowers at station A [27], particularly those most often involved in non-local mixing (oweniid and maldanid polychaetes). Biodiffusion was highest in May at stations B and C and was a factor of greater than 4 lower during the rest of the year. POC, chla, biogenic silica and fecal pellet fluxes, indicators of deposition of organic material, were considerably (2–15×) higher in May than during other seasons at these stations [25], and likely led to enhanced biodiffusion activities, as observed in other studies [15,41].
Diversity and size-structure of meio- and macrobenthic communities are similar through the year at each station [27], probably due to sedimentary OM remaining available during a large part of the year. Thus, it is supposed here that communities at each station change their bioturbation behaviour through the year depending on phytodetritus pulses and stock of degraded OM. Biodiffusion is dominant in May at the stations B and C but non-local (conveyer-like) mixing dominates from August to January. The quality of OM between May and August at the two stations may explain these changes, where sub-surface activities are dominant after rapid processing of the freshest phytodetritus.
Although we do not have bioturbation data in May and August at station A, bioturbation intensity there is lower than in stations B and C during the other seasons. This is caused by the impoverishment of benthic communities by conditions at the glacier front (hydrodynamics, erosion), although the sedimentary pigments are less degraded at this station from spring to fall. stations B and C have more diverse benthic communities and higher functional-trait diversity and bioturbation. This can explain why the two modes of bioturbation are observed at these two stations.
(c). Spatial and temporal differences in benthic fluxes
The remineralization of carbon and nutrients by Arctic seafloor communities has been shown to depend primarily on OM availability [41–45]. At deep Arctic basin stations, depth is as important as pigment concentration [33] but the depth range in this study was relatively narrow (80–305 m). SOD measured in this study is similar to other measurements of SOD from Kongsfjorden [42,46], and generally higher than colder Svalbard fjords [15,45,46] and deep-sea Arctic sediments [44]. SOD, stable over the year at each station, was higher at stations B and C where phaeobm and TOCbm were highest, although chla was higher at station A. The lack of significant seasonal variability in SOD at any of our stations is in direct contrast to findings in a number of empirical studies [8–11] showing SOD was linked with seasonal cycles of pelagic production. Only in deep areas such as the central Arctic basin, where the seasonal pelagic signals is likely reduced due to great depths and generally lower primary production, was no seasonality in SOD identified [7]. Response to pulsed food inputs can be realized in elevated SOD within days and persist for several weeks (e.g. [11]), and nutrient fluxes and bioturbation may respond at different time scales. Peak vertical flux of both POC and chla in 2012 was in May [25], simultaneous with the sediment flux and bioturbation measurements, so we would have expected a peak in one or more of these parameters if there was a response to seasonal inputs. In the other studies cited, however, the timing of sampling relative to a food pulse may influence whether a response is observed.
Maintaining high SOD at stations throughout the year necessitates storage of organic material within sediments and/or significant presence of other labile carbon sources. The presence of a 'food bank' within the sediment that is available for use throughout the year was the explanation for little seasonality in SOD observed along the western Antarctic Peninsula [47]. Alternatively, more than 40% of carbon assimilation by soft-sediment benthos in another boreal, ice-free fjord in west Svalbard came from macroalgal detritus, even at depths up to 400 m [48], and an average of 60% of sediment organic carbon in Kongsfjorden comes from macroalgae [49]. We have no reason to exclude either of these possibilities and, indeed, both may contribute to the lack of seasonality in SOD.
Fauna associated with higher bioturbation rates at stations B and C may also explain higher SOD [50]. The correlation of SOD with non-local transport might result from intensification of microbial processes in subsurface sediments at stations B and C caused by maldanid and spionid burrowing here [27]. Change in bioturbation mode between May and August at stations B and C is not perceptible in SOD. This may indicate either compensation in SOD between bioturbation functional groups and their associated microbial processes, or a dominance of maintenance respiration in fauna that overwhelms enhanced seasonal activities by diffusers or non-local mixers. Higher non-local transport in August likely stimulates the release of silicate to the overlying water and enhances sedimentary silicate dissolution [51]. Silicate dissolution, usually most efficient on grazed OM [52], is higher in stations with high phaeobm and enriched in diatoms. Thus, sediment microbes can accelerate biogenic silica dissolution by colonizing and enzymatically degrading the organic coating of diatom frustules.
Nutrient fluxes (in the same range of values from the Svalbard area [46] and in the Canadian Arctic [53]) indicate that fjord sediments are an important source of inorganic nutrients for driving primary production in the fjord. The seasonality of nutrient fluxes is further evidence of rapid processing of the sedimenting spring bloom/fecal pellet carbon. Higher release at station A might be the result of faster mineralization of the fresher sediment organic material, compared to at stations B and C, where degraded OM slows microbial mineralization rates and perhaps leads to denitrification at the outer station.
(d). Seasonality in a changing Arctic
Whereas strong seasonality of pelagic production and export of OM persists in a warmer, ice-free Kongsfjorden, the benthic system does not show a dramatic response. Such seasonality in Arctic benthic processes is most often observed in open shelf systems [8]. In one of the very few seasonal studies of benthic processes performed in fjords [15] a strong response to experimental food addition was observed in a high-Arctic system with up to 8 months of ice cover per year. We argue that the results from Kongsfjorden may be a model for many areas on Arctic shelves undergoing loss of sea-ice and warming waters. Thus, it is important to explore potential mechanisms for this loss of strong seasonality in some processes, and possible consequences for Arctic benthic ecology.
There is considerable observational evidence that reductions in sea-ice and warming temperatures are strongly linked to increased macroalgal production due in large part to increased light availability [54]. Macroalgae, particularly kelps, undergo seasonal growth and senescence cycles, generating considerable amounts of detritus [55]. Production of this detritus, however, is temporally distinct from pulses of pelagic phytodetritus as it arrives in autumn and winter due to natural senescence cycles and seasonal storms that detach living kelps from the sea floor. Increased light levels in a warming Arctic may also lead to enhanced growth of microphytobenthos, an as yet understudied source of OM to the coastal marine system [36]. Additionally, inputs of terrestrial carbon via rivers, melting glaciers, and coastal erosion are also predicted to increase considerably as the Arctic and surrounding systems warm [56]. It is already clear that much of this carbon is labile and is processed rapidly when it reaches the marine system [35], and is not restricted to coastal areas [57]. Thus, elevated levels and processing of detrital material in warming, ice-free fjords relative to cold, seasonally ice-covered fjords [48,49] suggest sediment communities in the future Arctic may function differently than at present.
How, then, will increased detritus content affect system functioning, aside from reduced seasonality? Detritus has been suggested to dampen seasonal fluctuations in carbon supply, leading to more stable food-chains and higher resilience to interannual variability in pelagic primary production [58]. Deposit-feeding taxa may be favoured over suspension-feeders in many of these systems with potential implications for bioturbation modes and intensity, and at a longer time scales, decreasing functional traits diversity within benthic communities. Monitoring programmes could target these questions in detailed comparative studies. Such food-web shifts have not been well studied but could have strong consequences for sustenance of top predators, including subsistence human populations. Finally, fjords have been identified as sites of disproportionately high carbon burial [23]. It is unclear whether increased detrital carbon and its impacts on seafloor processing will enhance or reduce this role. It is possible, however, that greater inputs of labile macroalgal detritus will enhance remineralization of more refractory sedimentary carbon that would otherwise be buried [59]. This 'organic priming' can be promoted by bioturbation, enabling fresher material and oxidants to reach buried carbon, and such processes demand both observational and experimental attention.
Responses to pulsed pelagic inputs will continue to be strong and pelagic–benthic coupling can still be quite tight, but the addition of detrital carbon from macroalgae, benthic microalgae and terrestrial sources can make it harder to track seasonality in benthic activity. Benthic processes may remain quite seasonal on oligotrophic shelves and ice-covered coastal regions, but increases in OM inputs from terrestrial and coastal systems may change this over time. The interaction of processes, including changes in relative inputs of different carbon sources, declining seasonality in benthic activity, and potential changes in bioturbation functional traits and food-web structure (i.e. decoupling of separate energy channels by enhancing detrital food web [60]), will likely result in ecosystem instability and in alteration of carbon cycling pathways. The potential consequences of fundamental changes in how OM is processed by Arctic benthos imply a need for the development of biomarkers and experimental approaches to tease apart these interactions.
Supplementary Material
Acknowledgements
We thank the crew of the MS Teisten, R/V Helmer Hanssen and the AWIPEV personnel in Ny-Ålesund for dedicated, professional assistance. We thank E. Amice, A. Aubert, J. Berge, S. Bourgeois, M. Calleja, E. Courtecuisse, G. Duong, C. Gueguen, P. Kerhervé, F. Narcy, J. Richard, V. Rissone and J. Søreide for their help with field sampling. The authors thank the two anonymous referees for the reviewing process.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
Substantial contributions to conception and design (N.M., E.M. and P.R.), acquisition of data (N.M., R.C. and P.R.), analysis and interpretation of data (N.M., E.M., M.A.P., J.D., M.L.G., R.C. and P.R.); (2) drafting the article or revising it critically for important intellectual content (N.M., E.M. and P.R.); and (3) final approval of the version to be published (N.M., E.M., M.A.P., J.D., M.L.G., R.C. and P.R.).
Competing interests
We declare we have no competing interests.
Funding
This study is a contribution to the projects ARCEx (NRC 228107), Marine Night (NRC 226417), WINBA (Arctic Field Grant), ESCOFAR (CNRS-EC2CO-DRIL and BIOHEFECT), IPEV (project no. 1132) and ECOTAB (ANR-11-PDOC-0018).
References
- 1.Berge J, et al. 2015. Unexpected levels of biological activity during the polar night offer new perspectives on a warming Arctic. Curr. Biol. 25, 2555–2561. ( 10.1016/j.cub.2015.08.024) [DOI] [PubMed] [Google Scholar]
- 2.Berge J, et al. 2015. In the dark: a review of ecosystem processes during the Arctic polar night. Prog. Oceanogr. 139, 258–271. ( 10.1016/j.pocean.2015.08.005) [DOI] [Google Scholar]
- 3.Berge J, Johnsen G, Cohen J, (eds) 2020. POLAR NIGHT marine ecology: life and light in the dead of night. Springer International Publishing. [Google Scholar]
- 4.Renaud PE, Ambrose WG, Węsławski JM. 2020. Benthic Communities in the Polar Night. In Polar night marine ecology: life and light in the dead of night (eds Berge J, Johnsen G, Cohen JH), pp. 161–179. Cham: Springer International Publishing. [Google Scholar]
- 5.Piepenburg D. 2005. Recent research on Arctic benthos: common notions need to be revised. Polar Biol. 28, 733–755. ( 10.1007/s00300-005-0013-5) [DOI] [Google Scholar]
- 6.Clough LM, Ambrose WG, Kirk Cochran J, Barnes C, Renaud PE, Aller RC. 1997. Infaunal density, biomass and bioturbation in the sediments of the Arctic Ocean. Deep Sea Res. Part II 44, 1683–1704. ( 10.1016/S0967-0645(97)00052-0) [DOI] [Google Scholar]
- 7.Oleszczuk B, Michaud E, Morata N, Renaud PE, Kędra M. 2019. Benthic macrofaunal bioturbation activities from shelf to deep basin in spring to summer transition in the Arctic Ocean. Mar. Environ. Res. 150, 104746 ( 10.1016/j.marenvres.2019.06.008) [DOI] [PubMed] [Google Scholar]
- 8.Bourgeois S, Archambault P, Witte U. 2017. Organic matter remineralization in marine sediments: a pan-Arctic synthesis. Global Biogeochem. Cycles 31, 190–213. ( 10.1002/2016GB005378) [DOI] [Google Scholar]
- 9.Grant J, Hargrave B, MacPherson P. 2002. Sediment properties and benthic–pelagic coupling in the North Water. Deep Sea Res. Part II 49, 5259–5275. ( 10.1016/S0967-0645(02)00189-3) [DOI] [Google Scholar]
- 10.Link H, Archambault P, Tamelander T, Renaud PE, Piepenburg D. 2011. Spring-to-summer changes and regional variability of benthic processes in the western Canadian Arctic. Polar Biol. 34, 2025–2038. ( 10.1007/s00300-011-1046-6) [DOI] [Google Scholar]
- 11.Renaud PE, Riedel A, Michel C, Morata N, Gosselin M, Juul-Pedersen T, Chiuchiolo A. 2007. Seasonal variation in benthic community oxygen demand: a response to an ice algal bloom in the Beaufort Sea, Canadian Arctic? J. Mar. Sys. 67, 1–12. ( 10.1016/j.jmarsys.2006.07.006) [DOI] [Google Scholar]
- 12.Rysgaard S, Thamdrup B, Risgaard-Petersen N, Fossing H, Berg P, Christensen PB, Dalsgaard T. 1998. Seasonal carbon and nutrient mineralization in a high-Arctic coastal marine sediment, Young Sound, Northeast Greenland. Mar. Ecol. Progress Ser. 175, 261–276. ( 10.3354/meps175261) [DOI] [Google Scholar]
- 13.Boetius A, et al. 2013. Export of algal biomass from the melting Arctic Sea ice. Science 339, 1430–1432. ( 10.1126/science.1231346) [DOI] [PubMed] [Google Scholar]
- 14.Morata N, Poulin M, Renaud PE. 2011. A multiple biomarker approach to tracking the fate of an ice algal bloom to the sea floor. Polar Biol. 34, 101–112. ( 10.1007/s00300-010-0863-3) [DOI] [Google Scholar]
- 15.Morata N, Michaud E, Włodarska-Kowalczuk M. 2015. Impact of early food input on the Arctic benthos activities during the polar night. Polar Biol. 38, 99–114. ( 10.1007/s00300-013-1414-5) [DOI] [Google Scholar]
- 16.Graf G. 1992. Benthic-pelagic coupling: a benthic view. In Oceanography and marine biology: an annual review, pp. 149–190, vol. 30. New York, NY: Routledge.
- 17.Grebmeier JM, Barry JP. 1991. The influence of oceanographic processes on pelagic-benthic coupling in polar regions: a benthic perspective. J. Mar. Sys. 2, 495–518. ( 10.1016/0924-7963(91)90049-Z) [DOI] [Google Scholar]
- 18.Petersen HG, Curtis MA. 1980. Differences in energy flow through major components of subarctic, temperate and tropical marine sheif ecosystems. Dana 1, 53–64. [Google Scholar]
- 19.Onarheim IH, Eldevik T, Smedsrud LH, Stroeve JC. 2018. Seasonal and regional manifestation of Arctic Sea Ice Loss. J. Clim. 31, 4917–4932. ( 10.1175/JCLI-D-17-0427.1) [DOI] [Google Scholar]
- 20.Wassmann P, Reigstad M. 2011. Future Arctic Ocean seasonal ice zones and implications for Pelagic-Benthic coupling. Oceanog 24, 220–231. ( 10.5670/oceanog.2011.74) [DOI] [Google Scholar]
- 21.Leu E, Mundy CJ, Assmy P, Campbell K, Gabrielsen TM, Gosselin M, Juul-Pedersen T, Gradinger R. 2015. Arctic spring awakening – steering principles behind the phenology of vernal ice algal blooms. Prog. Oceanogr. 139, 151–170. ( 10.1016/j.pocean.2015.07.012) [DOI] [Google Scholar]
- 22.Krause-Jensen D, Marbà N, Olesen B, Sejr MK, Christensen PB, Rodrigues J, Renaud PE, Balsby TJS, Rysgaard S. 2012. Seasonal sea ice cover as principal driver of spatial and temporal variation in depth extension and annual production of kelp in Greenland. Glob. Change Biol. 18, 2981–2994. ( 10.1111/j.1365-2486.2012.02765.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith RW, Bianchi TS, Allison M, Savage C, Galy V. 2015. High rates of organic carbon burial in fjord sediments globally. Nat. Geosci. 8, 450–453. ( 10.1038/ngeo2421) [DOI] [Google Scholar]
- 24.Pavlova O, Gerland S, Hop H. 2019. Changes in Sea-Ice Extent and Thickness in Kongsfjorden, Svalbard (2003–2016). In The ecosystem of Kongsfjorden, Svalbard (eds Hop H, Wiencke C), pp. 105–136. Cham: Springer International Publishing. [Google Scholar]
- 25.Lalande C, Moriceau B, Leynaert A, Morata N. 2016. Spatial and temporal variability in export fluxes of biogenic matter in Kongsfjorden. Polar Biol. 39, 1725–1738. ( 10.1007/s00300-016-1903-4) [DOI] [Google Scholar]
- 26.Hegseth EN, et al. 2019. Phytoplankton Seasonal Dynamics in Kongsfjorden, Svalbard and the Adjacent Shelf. In The ecosystem of Kongsfjorden, Svalbard (eds Hop H, Wiencke C), pp. 173–227. Cham: Springer International Publishing. [Google Scholar]
- 27.Włodarska-Kowalczuk M, Górska B, Deja K, Morata N. 2016. Do benthic meiofaunal and macrofaunal communities respond to seasonality in pelagial processes in an Arctic fjord (Kongsfjorden, Spitsbergen)? Polar Biol. 39, 2115–2129. ( 10.1007/s00300-016-1982-2) [DOI] [Google Scholar]
- 28.Mazurkiewicz M, Górska B, Renaud PE, Legeżyńska J, Berge J, Włodarska-Kowalczuk M. 2019. Seasonal constancy (summer vs. winter) of benthic size spectra in an Arctic fjord. Polar Biol. 42, 1255–1270. ( 10.1007/s00300-019-02515-2) [DOI] [Google Scholar]
- 29.Cottier FR, Nilsen F, Skogseth R, Tverberg V, Skarðhamar J, Svendsen H. 2010. Arctic fjords: a review of the oceanographic environment and dominant physical processes. Geol. Soc. Lond. Spec. Publ. 344, 35–50. ( 10.1144/SP344.4) [DOI] [Google Scholar]
- 30.Calleja ML, Kerhervé P, Bourgeois S, Kędra M, Leynaert A, Devred E, Babin M, Morata N. 2017. Effects of increase glacier discharge on phytoplankton bloom dynamics and pelagic geochemistry in a high Arctic fjord. Prog. Oceanogr. 159, 195–210. ( 10.1016/j.pocean.2017.07.005) [DOI] [Google Scholar]
- 31.Sokal RR, Rohlf F. 1995. Biometry. The principles and practice of statistics in biological research, 3rd edn New York, NY: WH Freeman & Co. [Google Scholar]
- 32.Lê S, Josse J, Husson F.. 2008. FactoMineR: an R Package for multivariate analysis. J. Stat. Softw. 25, 1–18. ( 10.18637/jss.v025.i01) [DOI] [Google Scholar]
- 33.Bourgeois S, Kerhervé P, Calleja MLI, Many G, Morata N. 2016. Glacier inputs influence organic matter composition and prokaryotic distribution in a high Arctic fjord (Kongsfjorden, Svalbard). J. Mar. Syst. 164, 112–127. ( 10.1016/j.jmarsys.2016.08.009) [DOI] [Google Scholar]
- 34.Kędra M, Kuliński K, Walkusz W, Legeżyńska J. 2012. The shallow benthic food web structure in the high Arctic does not follow seasonal changes in the surrounding environment. Estuarine, Coast. Shelf Sci. 114, 183–191. ( 10.1016/j.ecss.2012.08.015) [DOI] [Google Scholar]
- 35.Harris CM, McTigue ND, McClelland JW, Dunton KH. 2018. Do high Arctic coastal food webs rely on a terrestrial carbon subsidy? Food Webs 15, e00081 ( 10.1016/j.fooweb.2018.e00081) [DOI] [Google Scholar]
- 36.Woelfel J, Schumann R, Peine F, Flohr A, Kruss A, Tegowski J, Blondel P, Wiencke C, Karsten U. 2010. Microphytobenthos of Arctic Kongsfjorden (Svalbard, Norway): biomass and potential primary production along the shore line. Polar Biol. 33, 1239–1253. ( 10.1007/s00300-010-0813-0) [DOI] [Google Scholar]
- 37.Morata N, Renaud PE, Brugel S, Hobson KA, Johnson BJ. 2008. Spatial and seasonal variations in the pelagic–benthic coupling of the southeastern Beaufort Sea revealed by sedimentary biomarkers. Mar. Ecol. Prog. Ser. 371, 47–63. ( 10.3354/meps07677) [DOI] [Google Scholar]
- 38.Luckman A, Benn DI, Cottier F, Bevan S, Nilsen F, Inall M. 2015. Calving rates at tidewater glaciers vary strongly with ocean temperature. Nat. Commun. 6, 8566 ( 10.1038/ncomms9566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Promińska A, Cisek M, Walczowski W. 2017. Kongsfjorden and Hornsund hydrography – comparative study based on a multiyear survey in fjords of west Spitsbergen. Oceanologia 59, 397–412. ( 10.1016/j.oceano.2017.07.003) [DOI] [Google Scholar]
- 40.Holmes FA, Kirchner N, Kuttenkeuler J, Krützfeldt J, Noormets R.. 2019. Relating ocean temperatures to frontal ablation rates at Svalbard tidewater glaciers: insights from glacier proximal datasets. Sci. Rep. 9, 9442 ( 10.1038/s41598-019-45077-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerino M, Aller RC, Lee C, Cochran JK, Aller JY, Green MA, Hirschberg D. 1998. Comparison of different tracers and methods used to quantify bioturbation during a spring bloom: 234-thorium, luminophores and chlorophylla. Estuarine, Coast. Shelf Sci. 46, 531–547. ( 10.1006/ecss.1997.0298) [DOI] [Google Scholar]
- 42.Hulth S, Blackburn TH, Hall POJ. 1994. Arctic sediments (Svalbard): consumption and microdistribution of oxygen. Mar. Chem. 46, 293–316. ( 10.1016/0304-4203(94)90084-1) [DOI] [Google Scholar]
- 43.Boetius A, Damm E. 1998. Benthic oxygen uptake, hydrolytic potentials and microbial biomass at the Arctic continental slope. Deep Sea Res. Part I 45, 239–275. ( 10.1016/S0967-0637(97)00052-6) [DOI] [Google Scholar]
- 44.Cathalot C, Rabouille C, Sauter E, Schewe I, Soltwedel T. 2015. Benthic oxygen uptake in the Arctic Ocean margins - a case study at the deep-sea observatory HAUSGARTEN (Fram Strait). PLoS ONE 10, e0138339 ( 10.1371/journal.pone.0138339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kotwicki L, Grzelak K, Opaliński K, Węsławski JM. 2018. Total benthic oxygen uptake in two Arctic fjords (Spitsbergen) with different hydrological regimes. Oceanologia 60, 107–113. ( 10.1016/j.oceano.2017.11.005) [DOI] [Google Scholar]
- 46.Glud RN, Holby O, Hoffmann F, Canfield DE. 1998. Benthic mineralization and exchange in Arctic sediments (Svalbard, Norway). Mar. Ecol. Prog. Ser. 173, 237–251. ( 10.3354/meps173237) [DOI] [Google Scholar]
- 47.Smith CR, Mincks S, DeMaster DJ. 2006. A synthesis of bentho-pelagic coupling on the Antarctic shelf: food banks, ecosystem inertia and global climate change. Deep Sea Res. Part II 53, 875–894. ( 10.1016/j.dsr2.2006.02.001) [DOI] [Google Scholar]
- 48.Renaud PE, Løkken TS, Jørgensen LL, Berge J, Johnson BJ. 2015. Macroalgal detritus and food-web subsidies along an Arctic fjord depth-gradient. Front. Mar. Sci. 2, 31 ( 10.3389/fmars.2015.00031) [DOI] [Google Scholar]
- 49.Zaborska A, Włodarska-Kowalczuk M, Legeżyńska J, Jankowska E, Winogradow A, Deja K. 2018. Sedimentary organic matter sources, benthic consumption and burial in west Spitsbergen fjords – signs of maturing of Arctic fjordic systems? J. Mar. Sys. 180, 112–123. ( 10.1016/j.jmarsys.2016.11.005) [DOI] [Google Scholar]
- 50.Michaud E, Desrosiers G, Mermillod-Blondin F, Sundby B, Stora G. 2005. The functional group approach to bioturbation: the effects of biodiffusers and gallery-diffusers of the Macoma balthica community on sediment oxygen uptake. J. Exp. Mar. Biol. Ecol. 326, 77–88. ( 10.1016/j.jembe.2005.05.016) [DOI] [Google Scholar]
- 51.Marinelli RL. 1992. Effects of polychaetes on silicate dynamics and fluxes in sediments: importance of species, animal activity and polychaete effects on benthic diatoms. J. Mar. Res. 50, 745–779. ( 10.1357/002224092784797566) [DOI] [Google Scholar]
- 52.Gallinari M, Ragueneau O, DeMaster DJ, Hartnett H, Rickert D, Thomas C. 2008. Influence of seasonal phytodetritus deposition on biogenic silica dissolution in marine sediments—potential effects on preservation. Deep Sea Res. Part II 55, 2451–2464. ( 10.1016/j.dsr2.2008.06.005) [DOI] [Google Scholar]
- 53.Link H, Chaillou G, Forest A, Piepenburg D, Archambault P. 2013. Multivariate benthic ecosystem functioning in the Arctic - benthic fluxes explained by environmental parameters in the southeastern Beaufort Sea. Biogeosciences 10, 5911–5929. ( 10.5194/bg-10-5911-2013) [DOI] [Google Scholar]
- 54.Weslawski JM, Wiktor J, Kotwicki L. 2010. Increase in biodiversity in the arctic rocky littoral, Sorkappland, Svalbard, after 20 years of climate warming. Mar. Biodiv. 40, 123–130. ( 10.1007/s12526-010-0038-z) [DOI] [Google Scholar]
- 55.Buchholz CM, Wiencke C. 2016. Working on a baseline for the Kongsfjorden food web: production and properties of macroalgal particulate organic matter (POM). Polar Biol. 39, 2053–2064. ( 10.1007/s00300-015-1828-3) [DOI] [Google Scholar]
- 56.Parmentier F-JW, et al. 2017. A synthesis of the arctic terrestrial and marine carbon cycles under pressure from a dwindling cryosphere. Ambio 46, 53–69. ( 10.1007/s13280-016-0872-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Magen C, Chaillou G, Crowe SA, Mucci A, Sundby B, Gao A, Makabe R, Sasaki H. 2010. Origin and fate of particulate organic matter in the southern Beaufort Sea – Amundsen Gulf region, Canadian Arctic. Estuar. Coast. Shelf Sci. 86, 31–41. ( 10.1016/j.ecss.2009.09.009) [DOI] [Google Scholar]
- 58.Norkko A, Thrush SF, Cummings VJ, Gibbs MM, Andrew NL, Norkko J, Schwarz A-M. 2007. Trophic structure of coastal Antarctic food webs associated with changes in sea ice and food supply. Ecology 88, 2810–2820. ( 10.1890/06-1396.1) [DOI] [PubMed] [Google Scholar]
- 59.Aller RC, Cochran JK. 2019. The critical role of bioturbation for particle dynamics, priming potential, and organic C remineralization in marine sediments: local and basin scales. Front. Earth Sci. 7, 157 ( 10.3389/feart.2019.00157) [DOI] [Google Scholar]
- 60.McMeans BC, Rooney N, Arts MT, Fisk AT. 2013. Food web structure of a coastal Arctic marine ecosystem and implications for stability. Mar. Ecol. Progress Ser. 482, 17–28. ( 10.3354/meps10278) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.