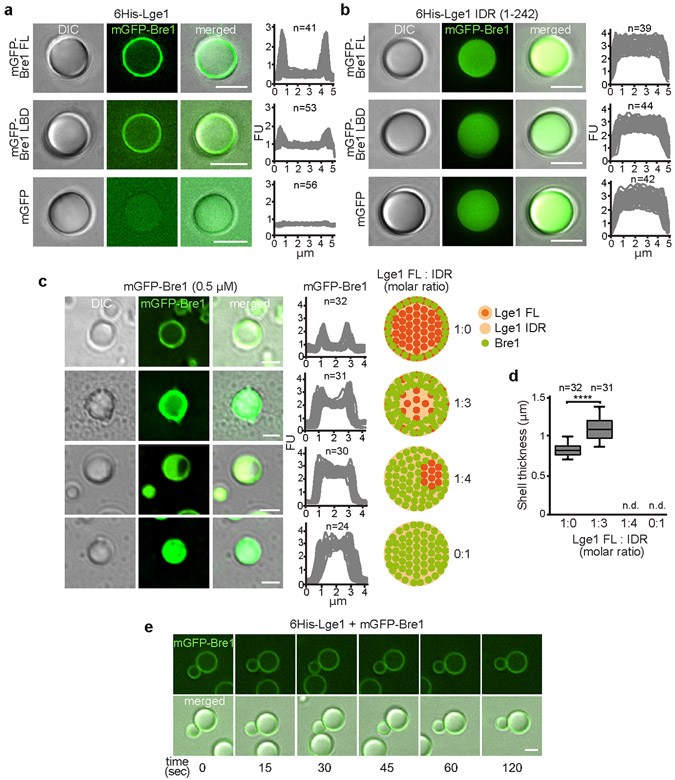
Extended Data Figure 3. Mechanism of Bre1 shell formation.
a-b. Reconstitution of condensates with core-shell architecture. Recombinant mGFP-tagged proteins (1.5 μM) were added to preformed 6His-Lge1 condensates or 6His-Lge1 IDR. Samples were incubated for 15 min prior to imaging by DIC and fluorescence microscopy. Scale bar, 2 μm. c. Reconstitution of ‘hybrid condensates’ with varying ratios of 6His-Lge1 FL: 6His-Lge1 IDR show differential partitioning of mGFP-Bre1 into the core. Proteins were mixed at the indicated molar ratios and incubated for 15 min at 20 °C. mGFP-Bre1 (0.5 μM) was added to the preformed condensates and incubated for 15 min prior to imaging by DIC and fluorescent microscopy. Fluorescent intensities were quantified across single condensates. Cartoons indicate putative assembly state of ‘hybrid condensates’, with a deterioration of the core-shell structure upon reduction of available Lge1 CCs. FU, arbitrary fluorescent units. n = number of condensates. Scale bar, 2 μm. d. Quantification of mGFP-Bre1 shell thickness in c. Box-whisker plot shows median, interquartile range, minimum and maximum values. ****p-value < 0.0001 determined with two-sided T-test (t= 9.4, df= 62). n = number of condensates. n.d., not determinable. e. Analysis of condensate fusion. 6His-Lge1 condensates (1.5 μM) with an mGFP-Bre1 shell (1.5 μM) were followed over time by microscopy. Condensates collide but do not fuse. Compare to Extended Data Fig. 1h. Scale bar, 2 μm. See also Video S2.