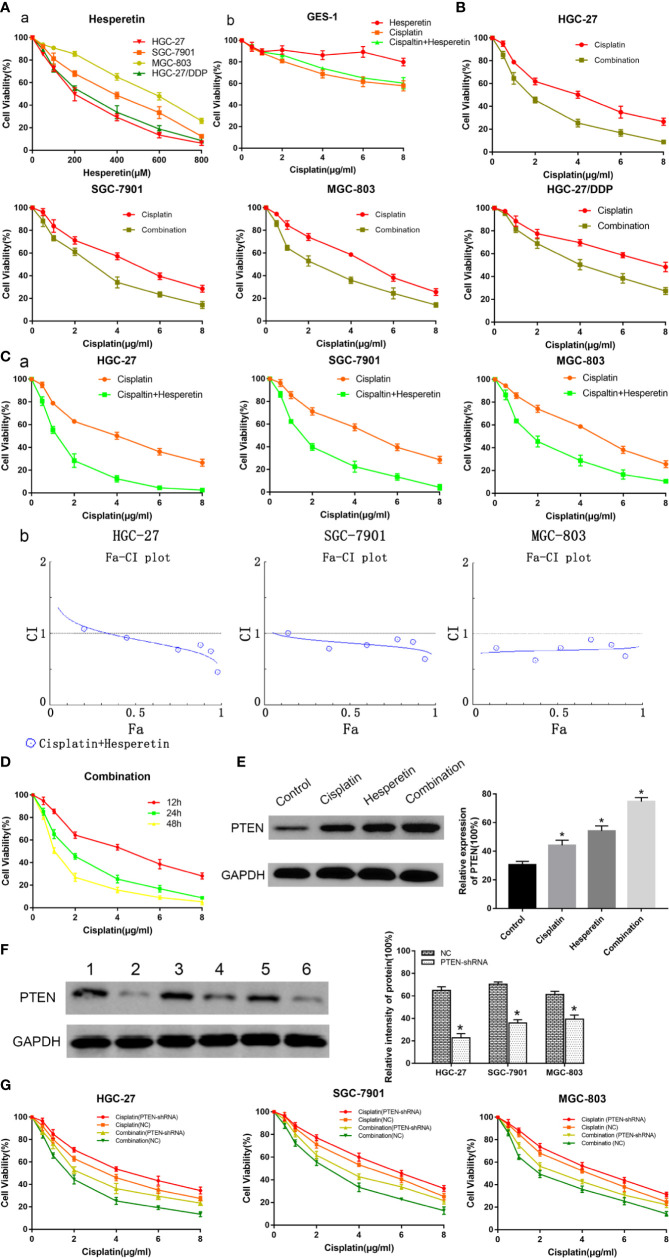
Figure 1.
Evaluation of the inhibitory effect of hesperetin, or cisplatin, or both, on the growth of GC cells using a CCK-8 kit. (A) a. GC cells (HGC-27, SGC-7901, MGC-803, and HGC-27/DDP) were treated with hesperetin (0, 50, 100, 200, 400, 600, and 800 µM). b. GES-1 cells were treated with cisplatin (0, 0.5, 1, 2, 4, 6, and 8 µg/ml), the different concentrations of hesperetin as described above and a combination of cisplatin and hesperetin for 24 h. (B) GC cells were treated with cisplatin and a combination of cisplatin and hesperetin (50 µM) for 24 h, respectively. (C) a. GC cells were treated with cisplatin and a combination of cisplatin and hesperetin (0, 50, 100, 200, 400, 600, and 800 µM) as described above for 24 h, respectively. b. Compusyn software was used to define the type of drug-combination effect. (D) HGC-27 cells were treated with the same concentration of cisplatin combined with hesperetin for 12, 24, or 48 h. (E) HGC-27 cells were incubated with the control, 4 μg/ml cisplatin, 200 μM hesperetin, or 4 µg/ml cisplatin + 200 μM hesperetin, and the levels of PTEN in GC cells were measured using western blotting. (F) The transfection efficiency of PTEN in GC cells was identified using western blotting. Lane 1 HGC-27/NC; Lane 2 HGC-27/PTEN-shRNA; Lane 3 SGC-7901/NC; Lane 4 SGC-7901/PTEN-shRNA; Lane 5 MGC-803/NC; Lane 6 MGC-803/PTEN-shRNA. *P < 0.05 vs. the corresponding negative control (NC) groups. (G) Transfected GC cells were treated with cisplatin (0, 0.5, 1, 2, 4, 6, and 8 µg/ml), and a combination of cisplatin and hesperetin (50 µM) as described above for 24 h, respectively. All the above data are shown as the mean ± SD from an average of three experiments.