Abstract
Introduction: This study was aimed to evaluate the beneficial effects of containing glucose tolerance factor (GTF) from Brewer's yeast in improving glycaemic control in diabetic rats and lowering some of the risk factors for cardiovascular disorders.
Methodology: The study used Wistar rats of both sexes weighing 150-200 g. Animals were randomly (n=6) divided into eight groups as normal control, normal control receiving Brewer's yeast, diabetic, Brewer's yeast receiving diabetic, Metformin-treated diabetic, and Glimepiride-treated diabetic, and two diabetic groups treated with Brewer's yeast and 50% of the Metformin and Glimepiride doses, respectively. To induce diabetes, Streptozotocin was administered intraperitoneally to all rats, except control group rats. Body weight (weekly), food intake (every day), blood glucose, and lipid profile (initially and at the end of the study) were assessed for four weeks in all groups.
Results: Brewer's yeast administration significantly decreased blood glucose levels and prevented reduction in body weight, increased food intake and alterations in the lipid profile compared to untreated groups, and were comparable to the groups treated with standard drugs.
Conclusion: Results of this study showed that oral administration of Brewer's yeast extract might be an excellent alternative antidiabetic agent which could be also useful in reducing the required dose of standard antidiabetic agents when combined.
Keywords:Brewer's yeast, diabetes mellitus, glucose tolerance factor (GTF), Streptozotocin.
INTRODUCTION
Diabetes mellitus is characterised by chronic hyperglycemia with carbohydrate, fat, and protein metabolism disorder arising from insulin secretion malfunction, its impact, or both (1).
Patients with diabetes mellitus can present both acute and chronic complications. Severe complications include ketoacidosis and ketoacidotic coma. Generally, chronic complications are classified into macrovascular and microvascular complications. Congestive cardiac failure, myocardial ischemia, and stroke represent over 70% of diabetic mortality, which belongs to macrovascular diseases. Also, diabetes has an increased risk of stroke, a significant cause of morbidity and mortality in diabetic patients (2). Previously conducted studies (3, 4) showed that the risk for stroke with high morbidity was significantly elevated in patients with type 1 or 2 diabetes. Increased blood glucose levels are typical in early stages of stroke, and a glucose level of more than 155 mg/dL within 48 hours after stroke onset is associated with a high mortality risk (5). Cardiovascular complications, including myocardial infarction, is one of the significant causes of death in diabetic patients. Diabetic cardiomyopathy is characterized by abnormal changes in morphology and function of the myocardial and coronary vasculature (6).
Presently, insulin and oral antidiabetic medications are the cornerstones of therapy, but they have their drawbacks. Hypoglycemia is commonly associated with higher doses of sulfonylureas, weight gain (sulfonylureas and meglitinides), hepatic dysfunction, increased heart failure risk as well as bone fractures (thiazolidinediones) and gastrointestinal disorders (acarbose) (7). Treatment for diabetes mellitus aims to allow patients to lead a normal life while maintaining a stable metabolic state to slow down or avoid long-term diabetes complications. There has been an ongoing search for new efficient, safer and cheaper drugs to achieve these goals. More attention has been given in recent years to the beneficial role of trivalent chromium designated an essential mineral in controlling the action of insulin and its effect on carbohydrate, protein, and lipid metabolism (8).
There is evidence that the marginal dietary intake of chromium in humans over decades can lead to a depletion of chromium content in the body. In the US, where the consumption of chromium is generally low, many older people may be affected. In some cases of maturity-onset diabetes and atherosclerotic disease, such a chromium deficiency was proposed as a contributing factor. Improved glucose tolerance and lower blood cholesterol occur in about 50 percent of the elderly subjects who are supplemented daily with inorganic chromium of 150-250 µg. There were also few experiments using Brewer's yeast as a glucose tolerance factor (GTF) – a complement to chromium. To date, no human studies have included control or placebo groups, and only one has quantified the amount of chromium being given.
Glucose tolerance factor is a dietary substance first obtained from Brewer's yeast, a natural compound that can correct diminished glucose tolerance in diabetic rats. It can be extracted from different sources, including liver, black pepper, and kidneys (9). Brewer's yeast is made from Saccharomyces cerevisiae, a single-celled fungus that is used to produce beer. It has been developed and used for years as a nutritional supplement. Brewer's yeast is a rich source of minerals, especially selenium, protein, B-complex vitamins, and chromium, a vital mineral trace that helps the body maintain normal levels of blood sugar (10).
Fischer and his group (8) investigated the effect of GTF on glucose transport in isolated cardiomyocytes, obtained via partial purification of yeast extract. In the absence of insulin, they found that GTF samples increased the rate of glucose transport in isolated cells 2- to 2,5-fold. Hwang et al (11) demonstrated an increase in 14C-glucose oxidation to CO₂ in rat adipocytes by adding several fractions derived from yeast.
The present hypothesis is that, in good responders, reduced glucose tolerance may be associated with insulin resistance associated with a deficiency in nutritional chromium. Given the growing interest in this subject, we investigated the effect of Brewer's yeast on experimentally induced diabetic rats.
For the present study, we selected Streptozotocin- induced diabetes in rats, as animal models provide crucial insight into the human diabetic disease. The majority of available models are rodent based because they are small, easy to handle, and economically advantageous and have a short interval of generation.
MATERIALS AND METHODS
Study design: experimental animal-based study.
Study design: experimental animal-based study. Study setting: Department of Pharmacology, BLDE (Deemed to be University), Sri B.M. Patil Medical College Hospital & Research Center, Vijayapur, Karnataka, India.
Study drugs: 1) Brewer's yeast 160 mg/kg bw/day orally, purchased from Blue Bird Foods (INDIA) PVT. LTD. Mumbai; 2) Streptozotocin (STZ) 60 mg/kg bw intraperitoneally, purchased from Rajesh Chemicals, Mumbai [CAS (1883-66-4)]; 3) Glimepiride 4 mg/kg bw/day orally, supplied by INDOCO Remedies LTD, Navi Mumbai; and 4) Metformin 180 mg/kg bw/day orally, supplied by USV Limited, Mumbai.
Study animals: Adult Wistar albino rats of either sex weighing 150-200 g, collected from the Sri BM Patil Medical College Hospital & Research Center in the Central Animal House, Vijayapur, Karnataka, India. Animals were individually housed in a quarantine room, in polypropylene cages, for one week, for acclimation before the beginning of the study. Cages filled with paddy husk that was replaced regularly were kept under a standard condition of illumination with a 12 h light-dark cycle at room temperature of 25±1° C and 45-70% relative humidity. Animals received commercial rat pellet chow (produced by VRK Nutritional solutions, Sangli, Maharashtra) and were granted access to water ad libitum.
Ethical clearance: The study was reviewed by the Institutional Animal Ethics Committee (IAEC) and approved by its vide letter reference number; 793/14, dated 04.12.2014, being conducted in compliance with the recommendations of the Committee for the Purpose of Control and Supervision of Experimentation on Animals (CPCSEA).
Grouping of animals: Animals were divided randomly into eight groups, with six individuals per group, as follows: group 1 (normal control) with gum acacia emulsion 0.5 mL per orally (PO) for four weeks; group 2 (normal control with Brewer's yeast extract treatment) with Brewer's Yeast emulsion 160 mg/kg bw/day PO for four weeks (12); group 3 [diabetic (STZ induced) control] with gum acacia emulsion 0.5 mL PO for four weeks (13); group 4 [diabetic rats (STZ induced) with Brewer's yeast extract] with Brewer's yeast 160 mg/kg bw/day PO for four weeks; group 5 [diabetic rats (STZ induced) with Glimepiride] with Glimepiride 2 mg/kg bw/day PO four weeks (14); group 6 [diabetic rats (STZ induced) + 50% of Glimepiride dose + 50% of Brewer's yeast dose] with Glimepiride 1 mg/kg bw for four weeks and Brewer's yeast 80 mg/kg bw/day PO four weeks; group 7 [Diabetic rats (STZ induced) with Metformin control] with Metformin (180 mg/kg bw/day) PO four weeks (15); and group 8 [diabetic rat (STZ induced) + 50% of Metformin dose + 50% of Brewer's yeast dose] with Metformin 90 mg/kg bw/day and Brewer's yeast 80 mg/kg bw/day PO four weeks. At the end of the study, no animal was sacrificed.
Dose calculation: Rat dose per day was calculated using the average human dose/day and converted into the equivalent rat dose using the following formula (16): Rat dose/200 g = human dose x 0.18.
Streptozotocin-induced diabetes: Streptozotocin was administered intraperitoneally in the dose of 60 mg/kg of the body weight. Streptozotocin induces diabetes within 3 to 10 days by destroying the beta cells (17). Rats were given STZ, while normal control rats were individually and separately kept in metabolic cages under feeding and metabolic control. Rats that showed an increase in blood glucose levels after three weeks of STZ administration were included in the diabetic group. Food consumption was measured in grams on a daily basis.
Laboratory parameters included: 1) body weight (day 0, 7, 14, 21 and 28); 2) food consumption (every day); 3) blood glucose levels, serum insulin levels, and serum lipid profile comprising serum cholesterol, triglycerides, and high-density lipoprotein were estimated initially and at the end of the study.
Statistical analysis. All values were calculated as mean±SEM and evaluated through a paired Student t-test and a one-way variance analysis (ANOVA) to test differences between groups. The level of statistical significance was set to p<0.05.
RESULTS
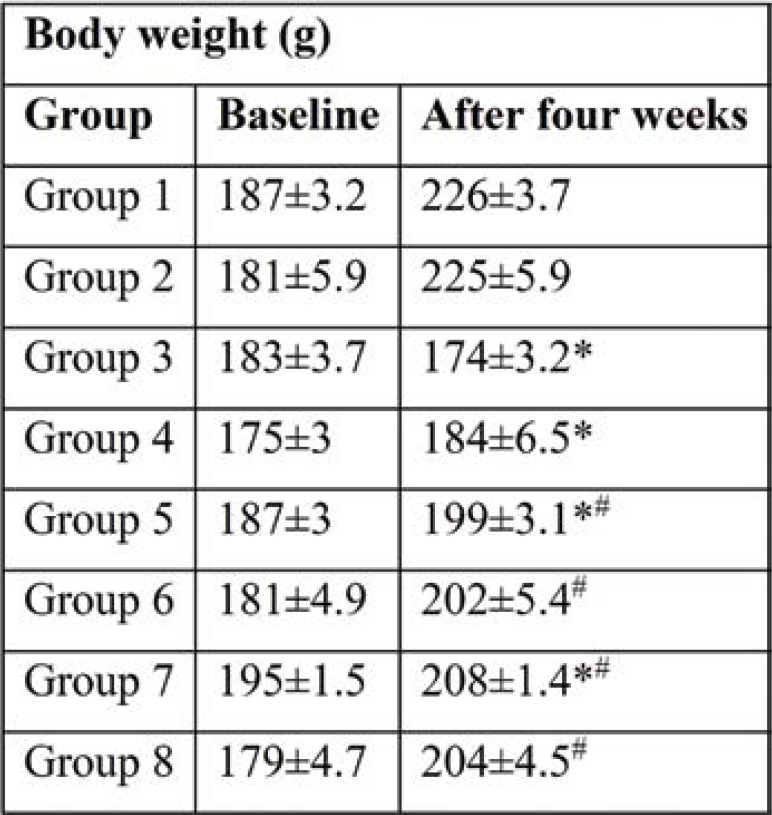
Body weight was increased in all groups. However, diabetic rats (group 3) showed a significant reduction in body weight after four weeks compared to those in the untreated control group. Rats in group 4 (diabetic rats), which were treated with Brewer's yeast, prevented a decrease in body weight significantly (p<0.05) and were comparable to untreated control rats. Diabetic rats (groups 5 and 7) treated with Glimiperide, and Metformin, respectively, had increased body weights significantly (p<0.05) and was comparable to the control group. Similarly, gain in body weight was also found significantly (p<0.05) in groups 6 and 8 compared to group 3. Treatment with half the dose of Glimiperide + Brewer's yeast and half dose of Metformin + Brewer's yeast, respectively prevented a decrease in body weight and were comparable to groups 5 and 7.
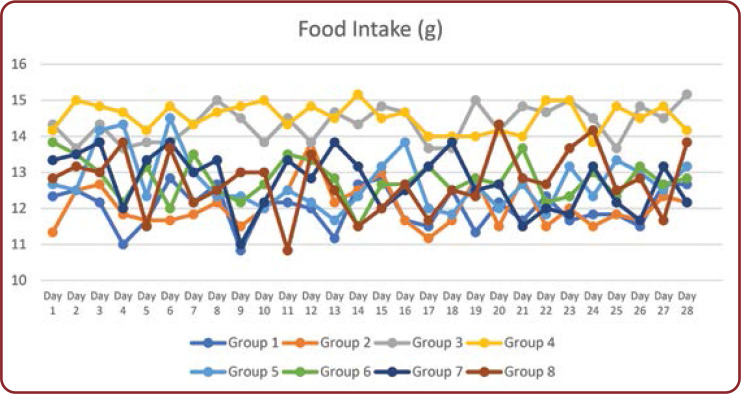
Food intake was monitored in all animals daily from day one to the end of the study (Figure 1). There was a significant (p<0.001) increase in food intake in untreated diabetic rats (group 3) compared to control animals. Administration of Brewer's yeast prevented this increase and was comparable to untreated controls. Treatment with Glimperide and Metformin (groups 5 and 7, respectively) and in combination with Brewer's yeast resulted in a decrease in food intake similar to control group rats. However, there were no significant differences.
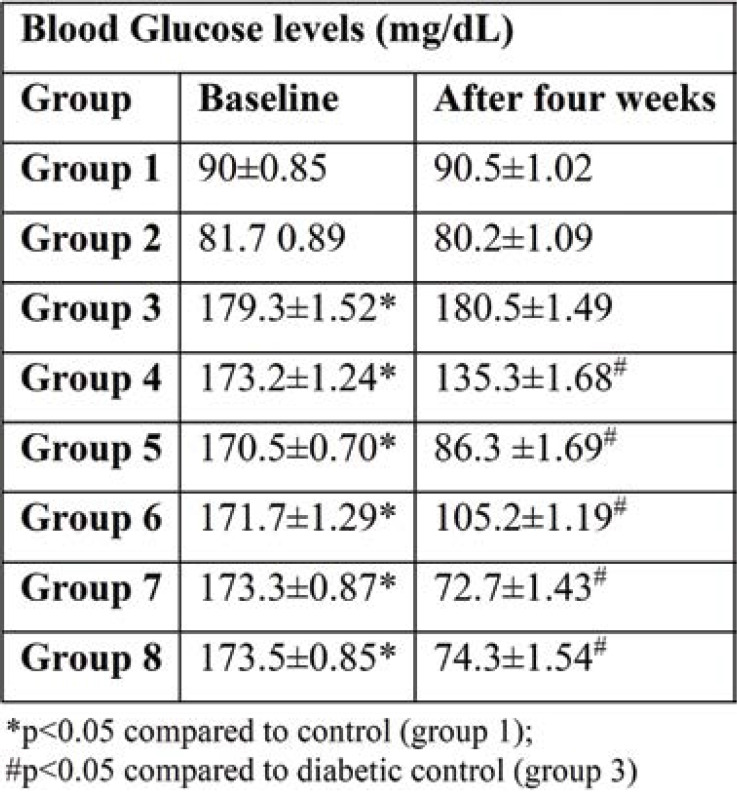
Blood glucose was measured initially and at the end of the study, and also every week (days 7, 14, and 21) by glucometer. Diabetic rats induced by STZ (Table 2) showed a significant (p<0.001) increase in blood glucose levels compared to normal control groups from the beginning of the study to the end. Treatment with Brewer's yeast alone in group 4 (diabetic rats + Brewer's yeast) showed a significant (p<0.05) reduction in blood glucose levels compared to untreated diabetic rats. However, blood glucose levels did not return to normal and were not comparable to normal control rats. Treatment with standard test drugs viz, Glimiperide and Metformin (groups 5 and 7, respectively) and half doses of these drugs with Brewer's yeast (groups 6 and 8) showed a significant reduction in blood glucose levels and returned to normal range compared to untreated diabetic rats and were comparable with normal control rats.
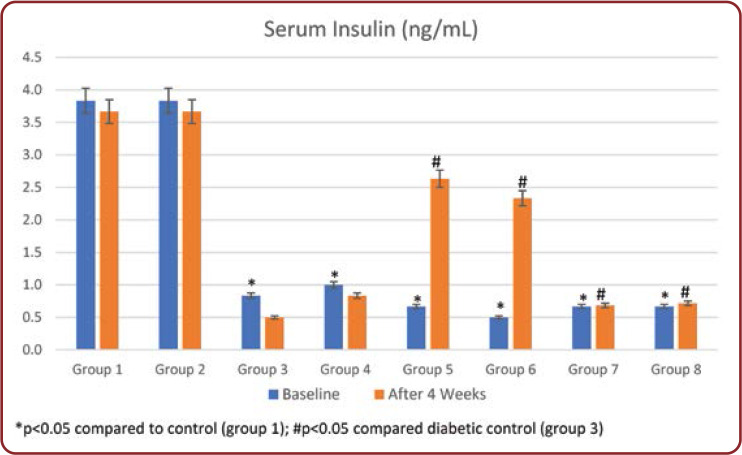
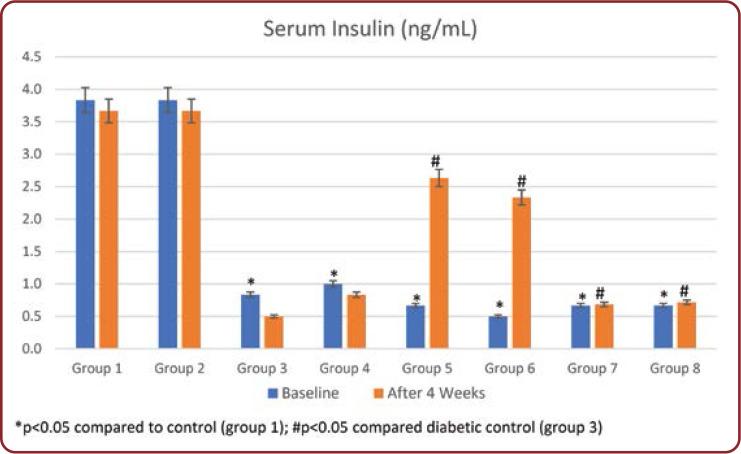
Serum insulin levels were measured initially and at the end of the study. In group 3, insulin levels were significantly decreased (p<0.001) compared to normal controls throughout the study period. Treatment with Brewer's yeast alone in group 4 showed a significant (p<0.05) increase in serum insulin levels compared to untreated diabetic rats. Similarly, standard drug treated rats (groups 5 and 7) and half dose of these drugs with Brewer's yeast (groups 6 and 8) showed a significant increase in serum insulin levels compared to untreated rats (group 3) and returned to normal range (Figure 2).
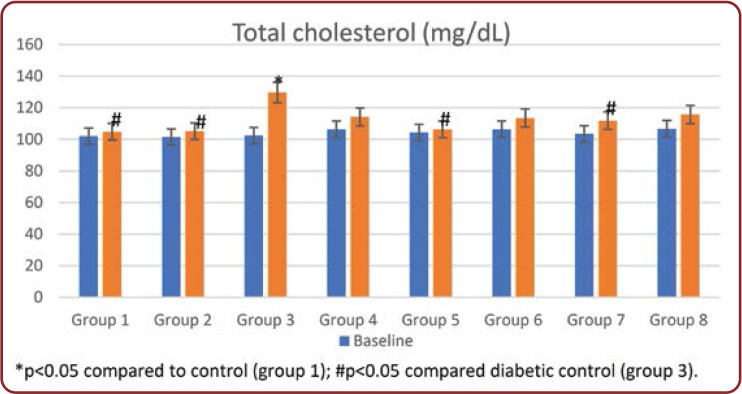
Over the study period, diabetic rats showed an alteration in serum total cholesterol (TC), LDL, triglycerides, and VLDL levels compared to those in a normal control group. These alterations in lipid profile were reduced by Brewer's yeast treatment (group 4) compared to untreated diabetic rats. Similarly, in rats treated with standard drugs and in combination with Brewer's yeast, these values returned to the normal range compared to untreated diabetic rats and were comparable to normal controls (Figures 3-5, Table 3).
DISCUSSIONS
The present study results revealed that the body weights in all groups, except group 3 (diabetic control) were significantly higher than those in group 1 at the end of the study (after four weeks). The significantly highest increase in body weight among all treated groups at the end of 28 days was observed in groups 3 and 4 as compared to the untreated diabetic group. Among treated groups, groups 3 and 4 showed a decrease in body weight at the end of the 28ͭͪ day compared to a healthy control group (Table 1). Diabetic rats’ drop in body weight was attributed to insulin deficiency resulting in reduced peripheral glucose use and excessive tissue protein breakdown (11). The liver, adipose tissue, and muscle have an improved catabolism response. The drop in body weight might be due to dehydration and catabolism of fats and proteins in diabetic rats. Increased catabolic reactions that lead to muscle wasting could also be the cause of weight loss in diabetic rats. Treatment with Glimepiride and Brewer's yeast (group 6) as well as treatment with Metformin and Brewer's yeast (group 8) resulted in an improved body weight, probably due to increased insulin secretion and enhance insulin sensitivity as well.
The serum glucose levels in diabetic controls (group 3) have been significantly higher over the whole experiment compared to other groups. At the end of the study, all groups 4-8 treated showed a significant reduction in glucose concentration compared with group 3. Treatment with the standard drug and Brewer's yeast combination groups 6 and 8 showed the highest decrease in blood glucose concentration during the study period among all treated groups (Table 2). This was further supported by an improvement in serum insulin levels of diabetic rats. Serum insulin levels in group 3 showed a significant decrease throughout the study period compared to normal controls (Figure 2). Administration of standard drugs and in combination with Brewer's yeast showed a significant improvement in serum insulin levels compared to diabetic controls.
Plasma levels of TC, TG, LDL increased, and HDL decreased in Streptozotocin-induced diabetic rats. These levels returned to normal in groups treated with Brewer's yeast extract alone and in combination with standard test drugs (groups 4-8) (Figures 3-5 and Table 3). The high levels of TC, TG, LDL, and decreased levels of HDL facilitate atherosclerosis which, combined with oxidative stress caused by hyperglycemia, are the risk factors for cardiovascular disease. This rise is due to an increased lipid breakdown and free fatty acid mobilization from peripheral depots. Because insulin inhibits the hormone-sensitive lipase, the latter becomes active in diabetes mellitus. In the serum of diabetic rats, excess fatty acids are converted into phospholipids and liver cholesterols. These two substances are discharged into the blood, along with excess TG in the liver.
These results showing an improvement of glucose tolerance with supplementation of chromium- rich yeast, are in accordance with several previous animal studies (18-21). In another study, tissue sensitivity to insulin diminished in experimental chromium deficiency (22). In rats, Mertz et al (23) showed that chromium improved the flow of glucose into tissues and chromium deficiency induced impaired glucose uptake and resistance to insulin. These can be returned to normal by chromium repletion (24). Others noticed genetically diabetic mice plasma glucose increases after GTF supplementation (25).
Chromium tends to be an essential trace element in controlling insulin action and its effects on carbohydrates, proteins, and lipid metabolism by increasing insulin sensitivity. Studies have shown that people with type 2 diabetes have low chromium compared with normal subjects, and chromium supplements boost insulin metabolic actions to increase glycaemic regulation. Insulin resistance with or without metabolic syndrome significantly raises the risk of cardiovascular diseases. By improving the chromium insulin sensitivity as a chromium picolinate decreases the risk of adverse cardiovascular events (26).
Chen WY et al (2009) studied the effect of chromium supplement in male KK/HIJ mice (genetically obese and insulin resistance) and analyzed functionally characterized insulin resistance, changes in blood biochemicals, inflammatory markers, insulin signaling molecules in skeletal muscle. The above-mentioned authors concluded that chromium supplementation could have a beneficial impact on diabetic subjects by enhancing insulin signaling in the skeletal muscle (27).
Payam Hosseinzadh et al (2013) carried out a randomized controlled clinical trial to assess the beneficial effect of Brewer's yeast supplement on glycemic indices in 84 adult type 2 diabetes mellitus patients, with supplement group receiving Brewer's yeast six 300 mg tablets/day, totally 1 800 mg, and placebo six 300 mg tablets/day for 12 weeks. They found that fasting blood sugar (FBS), glycosylated hemoglobin changes, and insulin sensitivity were significantly different between the two groups during the study, indicating that dietary supplements with Brewer's leaves might boost blood glucose variables in type 2 diabetes mellitus besides the regular diabetes treatment (12).
Bahijiri SM et al (2000) investigated the impact of organic and inorganic chromium supplementation on glucose tolerance, serum lipids, and drug dosage in patients with type 2 diabetes in order to find a safer and more economical glycemic control method. Their study showed that chromium supplementation provided a better control of glucose and lipid variables, while decreasing the dosage of the antidiabetic drugs, Glibenclamide in type 2 diabetes patients (28).
Grant AP et al (1982) performed a doubleblind cross-over study comparing placebo with the glucose tolerance factor (GTF) in the form of a brewer yeast supplement containing 1.28 ug chromium daily over a seven-week trial period. Treatment with Brewer's yeast (GTF) improved diabetic control, as demonstrated by a fall in HBA1c% (P<0.001) and a rise in HDL (p<0.05) (29).
Lia MH et al (2006) explored the ability of chromium yeast supplementation to enhance carbohydrate and lipid metabolism in Streptozotocin- induced diabetic rats. Their findings indicated that chromium yeast supplementation reduced fasting blood glucose and LDL-cholesterol levels in STZ-induced diabetic rats. This research increases the possibility of using the supplementation of chromium yeast to boost carbohydrate and lipid metabolism in patients with type 2 diabetes mellitus (30).
In one of the large-scale trials, Anderson et al (1997) used a placebo, 200 mcg or 1 000 mcg of chromium per day to test 180 subjects with type 2 diabetes mellitus. Two months later, HBA1c values were significantly increased in the group receiving 1 000 mcg of chromium a day, but after four months they were lower in both chromium groups. Fasting and insulin levels of two hours in both groups receiving chromium supplement decreased significantly after two and four months. These data showed that chromium supplements had critical beneficial effects on carbohydrate metabolism, as assessed in patients with type 2 diabetes mellitus with HBA1c, insulin, and glucose values (31).
Racek J et al (2006) investigated the effect of chromium-enriched yeast on blood glucose and insulin variables, blood lipids, and oxidative stress markers in individuals with type 2 diabetes mellitus. Chromium-enriched yeast supplementation was associated with a significant decrease in serum glucose compared to placebo (p<0.01), fasting serum insulin decreased further in the chromium group. These results suggest that chromium-enriched yeast supplementation in well-controlled type 2 diabetes mellitus is healthy and can lead to improvements in blood glucose variables and oxidative stress (32).
CONCLUSION
Our results show an increased glycemic control in diabetic rats and lipid metabolism, which decreases risk factors for coronary heart disease in diabetes mellitus. Chromium in Brewer's yeast appears to be involved in controlling the action of insulin and has an effect on carbohydrates, proteins, and lipid metabolism through insulin sensitivity.
Insulin resistance with or without metabolic syndrome significantly raises the risk of cardiovascular diseases. Through increasing the chromium insulin sensitivity as a chromium picolinate decreases the risk of adverse cardiovascular events. This research expands the possibility of using chromium-leaven supplements in patients with type 2 diabetes mellitus to boost carbohydrate and lipid metabolism.
Conflicts of interest: none declared.
Financial support: none declared.
Acknowledgements: The authors would like to thank the staff of the Department of Pharmacology, BLDE (Deemed to be) Universitys Shri BM Patil Medical College, for constant support. I also extend my thanks to my family members and friends who have helped me indirectly and supported me during my work.
TABLE 1.
Weekly body weight (g) in all groups (mean±SEM)
TABLE 2.
Blood glucose levels (mg/dL) in all groups (mean±SEM)
FIGURE 1.
Daily food intake (gms) in all groups
FIGURE 2.
Daily food intake (gms) in all groups
FIGURE 3.
Total cholesterol levels (mg/dL) in all groups
FIGURE 4.
Serum triglyceride levels (mg/dL) in all groups
FIGURE 5.
High-density lipoprotein levels (mg/dL) in all groups
TABLE 3.
Very low-density lipoprotein levels (mg/dL) in all groups (mean±SEM)
Contributor Information
Jyoti S. PATIL, Department of Pharmacology, BLDE (Deemed to be University), Shri BM Patil Medical College, Vijayapur, Karnataka, India
Akram A. NAIKAWADI, Department of Pharmacology, BLDE (Deemed to be University), Shri BM Patil Medical College, Vijayapur, Karnataka, India
Gurudatta MOHARIR, Department of Pharmacology, BLDE (Deemed to be University), Shri BM Patil Medical College, Vijayapur, Karnataka, India.
Ambadasu BHARATHA, Faculty of Medical Sciences, The University of the West Indies, Cave Hill Campus, Barbados, WI.
References
- 1.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. In: Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 2.Hyvärinen M, Qiao Q, Tuomilehto J, et al. Hyperglycemia and stroke mortality. Diabetes Care. 2009;2:348–354. doi: 10.2337/dc08-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: A risk profile from the framingham study. Stroke. 1991;3:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 4.Tuomilehto J, Rastenyte D, Jousilahti P, et al. Diabetes mellitus as a risk factor for death from stroke. Prospective study of the middle-aged Finnish population. Stroke. 1996;2:210–215. doi: 10.1161/01.str.27.2.210. [DOI] [PubMed] [Google Scholar]
- 5.Fuentes B, JoséCastillo, José BS, et al. The prognostic value of capillary glucose levels in acute stroke the GLycemia in acute stroke (GLIAS) study. Stroke. 2009;2:562–568. doi: 10.1161/STROKEAHA.108.519926. [DOI] [PubMed] [Google Scholar]
- 6.Sander GE, Wilklow FE, Giles TD. Heart failure in diabetes mellitus: causal and treatment considerations. Minerva Cardioangiol. 2004;52:491–503. [PubMed] [Google Scholar]
- 7.Min TS, Park SH. Therapy of Diabetes Mellitus Using Experimental Animal Models. Asian-Australasian J Anim Sci. 2010;23(5):672–679. [Google Scholar]
- 8.Moyad MA. Brewer’s/baker’s yeast (Saccharomyces cerevisiae) and preventive medicine: Part II. Urologic Nursing. 2008;1:73–75. [PubMed] [Google Scholar]
- 9.Mirsky N. Glucose Tolerance Factor-Insulin Mimetic and Potentiating Agent-A Source for a Novel Anti Diabetic Medication. Available from: http://dx.doi.org/10.5772/54350. 2012.
- 10.Lao L, Berman BM. The Center for Integrative Medicine at the University of Maryland: The first complementary and alternative medicine center in a US medical school. J Chinese Integr Med. 2008;11:1205–1209. doi: 10.3736/jcim20091120. [DOI] [PubMed] [Google Scholar]
- 11.Kamalakkannan N, Prince PSM. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic Wistar rats. Basic Clin Pharmacol Toxicol. 2006;1:97–103. doi: 10.1111/j.1742-7843.2006.pto_241.x. [DOI] [PubMed] [Google Scholar]
- 12.Hosseinzadeh P, Javanbakht MH, Mostafavi S-A, et al. Brewer’s Yeast Improves Glycemic Indices in Type 2 Diabetes Mellitus. Int J Prev Med. 2013;10:1131–1138. [PMC free article] [PubMed] [Google Scholar]
- 13.Akbar DH, Hagras MM, Amin HA, Khorshid OA. Comparison between the effect of glibenclamide and captopril on experimentally induced diabetic nephropathy in rats. J Renin Angiotensin Aldosterone Syst. 2013;2:103–115. doi: 10.1177/1470320312460881. [DOI] [PubMed] [Google Scholar]
- 14.Draeger KE, Wernicke-Panten K, Lomp HJ, et al. Long-term treatment of type 2 diabetic patients with the new oral antidiabetic agent climepiride (Amaryl®): A double-blind comparison with clibenclamide. In: Hormone and Metabolic Research. 1996. pp. 419–425. [DOI] [PubMed]
- 15.Gad MZ, Wahman LF. Pioglitazone versus Metformin in Two Rat Models of Glucose Intolerance and Diabetes. Pak J Pharm Sci. 2010;3:3015–312. [PubMed] [Google Scholar]
- 16.Ghosh MN, Pagets and Barnes. Table 25.2. In: Fundamentals of Experimental Pharmacology. 5th ed., India: Hilton & Company: 2011.
- 17.Damasceno DC, Netto AO, Iessi IL, et al. Streptozotocin-induced diabetes models: Pathophysiological mechanisms and fetal outcomes. [DOI] [PMC free article] [PubMed]
- 18.Mertz W. Effects and Metabolism of Glucose Tolerance Factor. Nutr Rev. 1975;5:129–135. doi: 10.1111/j.1753-4887.1975.tb07105.x. [DOI] [PubMed] [Google Scholar]
- 19.Davidson IWF, Blackwell WL. Changes in Carbohydrate Metabolism of Squirrel Monkeys with Chromium Dietary Supplementation. Proc Soc Exp Biol Med. 1968;1:66–70. doi: 10.3181/00379727-127-32622. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder HA. Chromium Deficiency in Rats: a Syndrome Simulating Diabetes Mellitus with Retarded Growth. J Nutr. 1966;4:439–445. doi: 10.1093/jn/88.4.439. [DOI] [PubMed] [Google Scholar]
- 21.Mertz W, Roginski EE, Schroeder HA. Some aspects of glucose metabolism of chromium deficient rats raised in a strictly controlled environment. J Nutr. 1965;87:107–110. doi: 10.1093/jn/86.1.107. [DOI] [PubMed] [Google Scholar]
- 22.Mertz W. Chromium and its relation to carbohydrate metabolism. Med Clin North Am - http://www.ncbi.nlm.nih.gov/pubmed/775215. 1976;4:739–744. doi: 10.1016/s0025-7125(16)31857-0. [DOI] [PubMed] [Google Scholar]
- 23.Mertz W, Roginski EE, Reba RC. Biological activity and fate of trace quantities of intravenous chromium (3) in the rat. Am J Physiol. 1965;3:489–494. doi: 10.1152/ajplegacy.1965.209.3.489. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz K, Mertz W. A glucose tolerance factor and its differentiation from factor 3. Arch Biochem Biophys. Available from: http://www.ncbi.nlm.nih.gov/pubmed/13479136. 1957. [DOI] [PubMed]
- 25.Tuman RW, Doisy RS. Studies in the genetically diabetic mouse: effect of GTF and clofibrate (CPIB) on the diabetic syndrome. In: Trace Element Metabolism in Animals. 1974. pp. 678–688.
- 26.Tuman RW, Bilbo JT, Doisy RJ. Comparison and effects of natural and synthetic glucose tolerance factor in normal and genetically diabetic mice. Diabetes. 1978;1:49–56. doi: 10.2337/diab.27.1.49. [DOI] [PubMed] [Google Scholar]
- 27.Chen WY, Chen CJ, Liu CH, Mao FC. Chromium supplementation enhances insulin signalling in skeletal muscle of obese KK/HlJ diabetic mice. Diabetes, Obes Metab. 2009;4:293–303. doi: 10.1111/j.1463-1326.2008.00936.x. [DOI] [PubMed] [Google Scholar]
- 28.Bahijiri SM, Mira SA, Mufti AM, Ajabnoor MA. The effects of inorganic chromium and brewer’s yeast supplementation on glucose tolerance, serum lipids and drug dosage in individuals with type 2 diabetes. Saudi Med J. 2000;9:831–837. [PubMed] [Google Scholar]
- 29.Grant AP, McMullen JK. The effect of brewers yeast containing glucose tolerance factor on the response to treatment in type 2 diabetics. A short controlled study. Ulster Med J. 1982;2:110–114. [PMC free article] [PubMed] [Google Scholar]
- 30.Lai MH, Chen YY, Cheng HH. Chromium yeast supplementation improves fasting plasma glucose and LDL-cholesterol in streptozotocin-induced diabetic rats. Int J Vitam Nutr Res. 2006;6:391–397. doi: 10.1024/0300-9831.76.6.391. [DOI] [PubMed] [Google Scholar]
- 31.Anderson RA, Cheng N, Bryden NA, et al. Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes. 1997;11:1786–1791. doi: 10.2337/diab.46.11.1786. [DOI] [PubMed] [Google Scholar]
- 32.Racek J, Trefil L, Rajdl D, et al. Influence of chromium-enriched yeast on blood glucose and insulin variables, blood lipids, and markers of oxidative stress in subjects with type 2 diabetes mellitus. Biol Trace Elem Res. 2006;3:215–230. doi: 10.1385/BTER:109:3:215. [DOI] [PubMed] [Google Scholar]