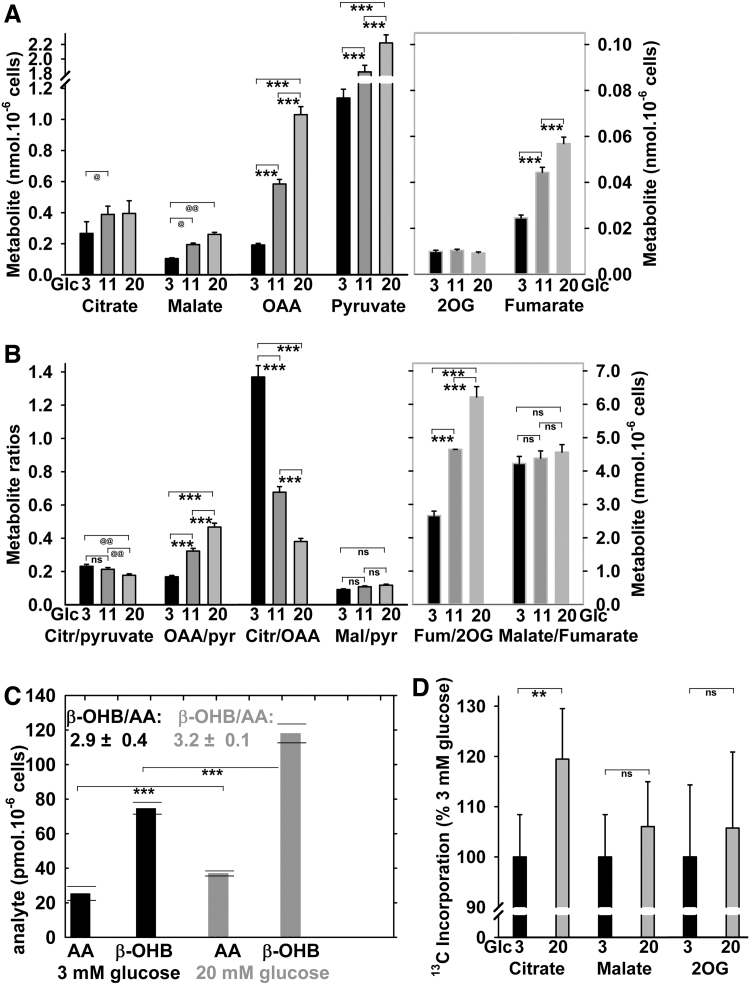
FIG. 6.
Targeted metabolomics of the Krebs cycle intermediates, acetoacetate, and β-OHB and evidence for the isocitrate/pyruvate redox shuttle. Total cellular metabolite levels (A), their selected ratios (B), and AA and β-OHB and their ratios as indicated (C) were quantified in INS-1E cells preincubated with 3 mM glucose for 1 h; then, glucose was raised by zero, 8, and 17 mM surplus to reach a final concentration of 3 mM (black bars), 11 mM (dark gray bars), and 20 mM (gray bars) glucose and subsequently incubated for another 30 min. ANOVA for (A, B) yielded (n = 5) ***p < 0.001; whereas Student's t-test for (A, B) yielded (n = 5): @@p < 0.05; @p < 0.1; and for (C) (all estimates from two independent experiments are shown with averages and SDs); p < 0.001 for all combinations between the two compounds. The difference between the β-OHB/AA ratios was not significant. Notably, the significant β-OHB increase suggests also the increase in mitochondrial matrix NAD+, since β-OHB dehydrogenase, which exists only in the mitochondrial matrix, produces β-OHB from AA at the expense of NADH, thus forming NAD+ (46). The fact that AA does not proportionally decrease reflects other reactions (43, 46) and penetration of AA into the cytosol during the sample preparation (Supplementary Fig. S2Fa). (D) 13C incorporation from 1-13C-l-glutamine into citrate, malate, and 2OG is expressed for normalized data for 25 mM glucose (2-h incubations) in relation to average values obtained after a 2-h incubation with 3 mM glucose. Data were first calculated in % of 13C accumulated amounts versus total (13C+12C) amount of a given compound when accounted for the natural 13C content. Evidence for the isocitrate/pyruvate redox shuttle is suggested by the existence of the 13C-accumulation, as such. This is because the 13C-accumulation into citrate from 1-13C-glutamine cannot exist on the forward Krebs cycle, since 13C-CO2 is formed and eliminated from the sample; hence any 13C-labeled citrate or malate molecule (when subtracting those with naturally occurring 13C) must originate from the reverse Krebs cycle direction, given by the IDH2-mediated NADPH-driven reductive carboxylation of 2OG. ANOVA for (D) (n = 6): **p < 0.05. Also, insignificantly (“ns”) increased 13C incorporation into malate and 2OG is indicated. β-OHB, β-hydroxybutyrate; AA, acetoacetate; ns, nonsignificant; SDs, standard deviations.