Figure 2.
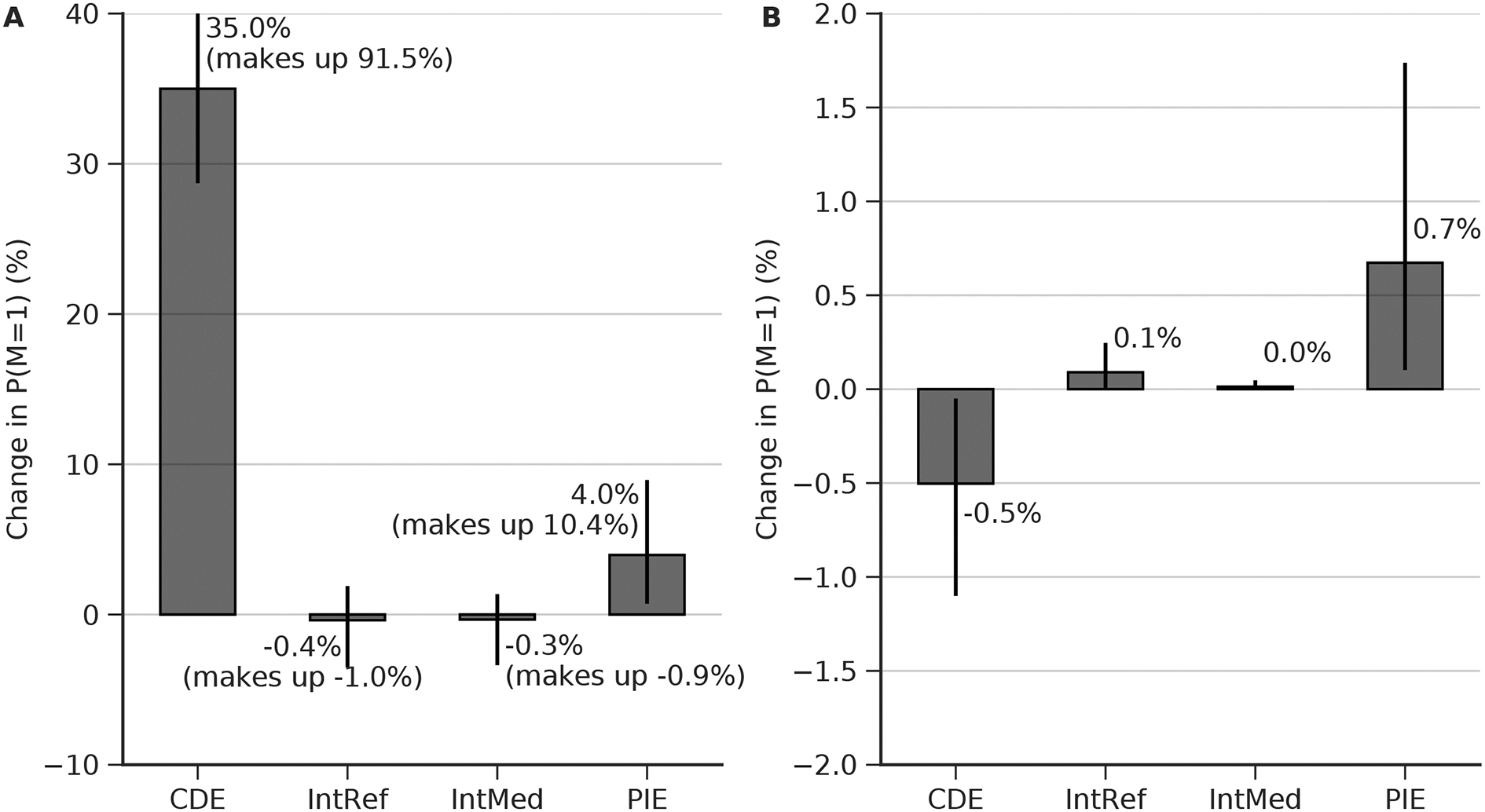
Estimated direct and mediated effects on mortality of burst suppression burden, clinical condition, and drug exposure. B: burst suppression burden measured by the percentage of 8–12 hour EEG epochs with burst suppression (see methods). M: in-hospital mortality (1, deceased; 0, alive). D: drug, i.e. cumulative propofol exposure. C: patient characteristics including demographics and critical illnesses (Table 1). The estimated effect is shown above each arrow, measured on the difference scale. The width of each arrow is proportional to the estimated effect. Effects are measure as the difference in expected outcome between groups in a counterfactual randomized clinical trial (cRCT), which two groups are randomized to two different levels of B, D, or C. (A) Difference in mortality between patients randomized to 0% vs 100% burst suppression burden. (B)Difference in mortality between patients randomized to high vs. low cumulative exposure to propofol. Direct (D->M) and indirect (D->B->M) effects are shown. (C) Difference in mortality between ICU patients randomized to severe vs mild critical illness severity. Direct (C->M) an indirect (C->B->M) effects are shown. (D) Sensitivity analysis, measuring the minimum risk ratio of unmeasured confounding factor to either the exposure or the outcome that would be needed to explain away the estimated effect (E-value). These results were obtained using random forest and doubly robust estimation.
