Figure 1.
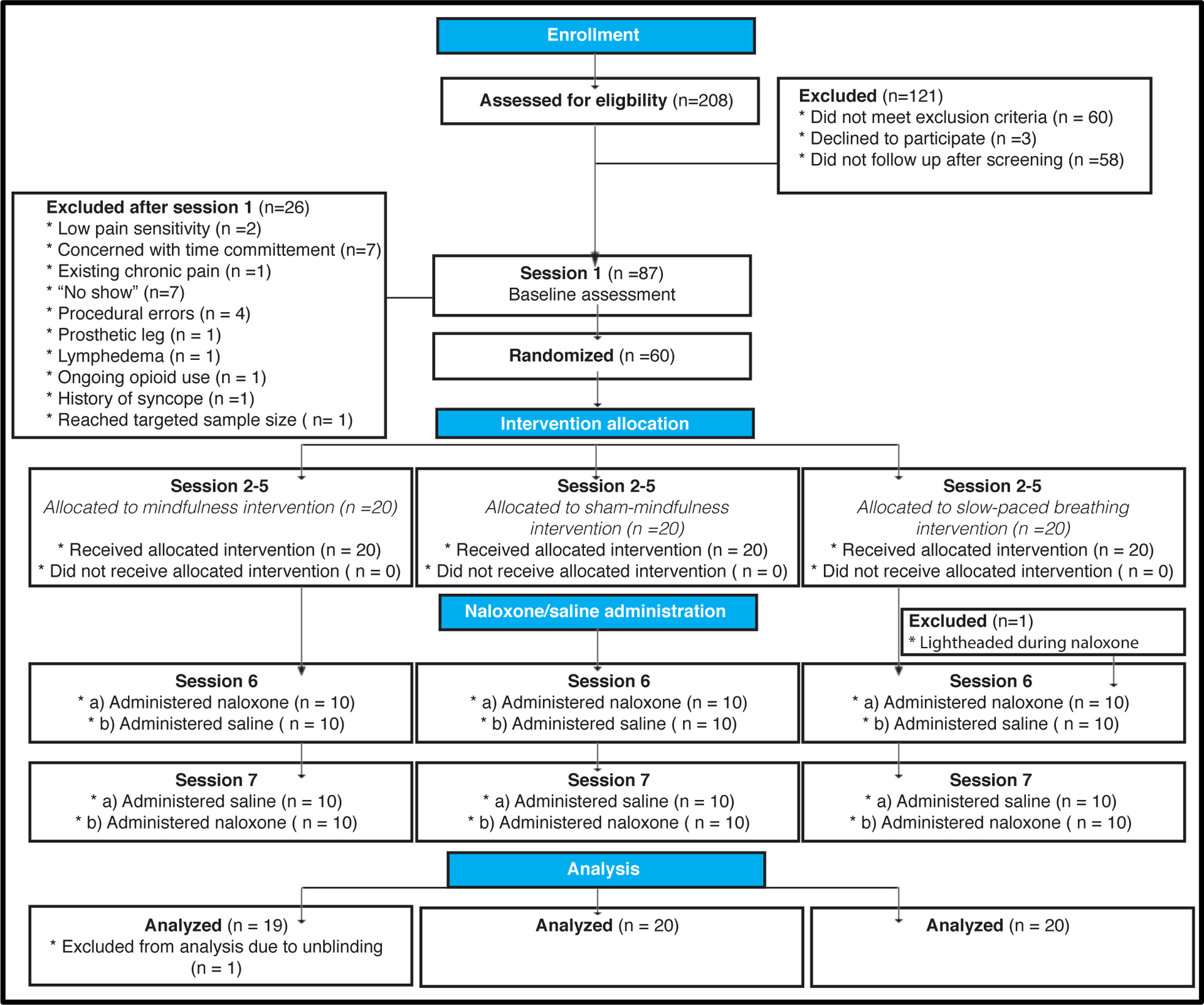
Experimental Design. The proposed mechanistically focused, crossover and double-blinded clinical trial included 7 separate sessions. After potential participants met study inclusion/exclusion criteria, participants completed study session 1, which served as a baseline control. After study session 1, 60 participants were randomized into one of three groups (mindfulness; sham-mindfulness; book-listening control). After their respective four-day interventions (Session 2–5), subjects participated in the post-intervention experimental session (session 6). Here, we assessed the effects of each respective manipulation during naloxone/saline infusion and noxious stimulation. One participant, in the slow-paced breathing group, was dismissed during naloxone infusion during session 6. Session 7’s experimental procedures were matched to session 6 except for the respective drug assignment. One participant from the mindfulness meditation group was dismissed from the final analysis because said subject’s drug assignment was unintentionally revealed and subsequently unblinded. Thus, a total of 59 subject’s data were included in the present study.
