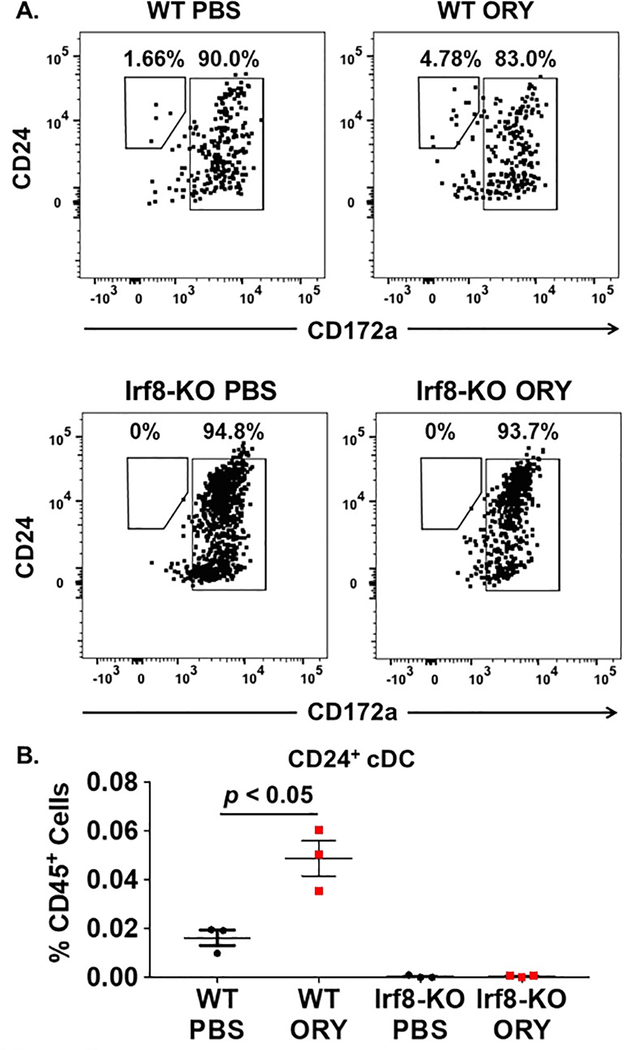
Figure 5. LSD1 inhibition requires IRF8 for effect on CD24+ cDC differentiation.
C-kit+ cells were isolated from mouse bone marrow and cultured on MS-5 stroma cells for 14 days in media supplemented with 10 ng/ml Flt3 ligand as described in the “Methods” section. Cultures were treated with PBS vehicle or ORY (0.1 μM) for the 1st 7 days of the culture period. Measurement of CD24+ cDC differentiation was performed using flow cytometry at the end of the culture period. CD24+ cDCs were defined by the immunophenotype mouse CD45+, lineage-negative (lin-), MHC-II+, CD11c+, CD24+, CD172-. (A) Flow cytometry analysis of CD24+ cDC populations following culture of wild-type (WT; top) or Irf8−/− (Irf8-KO; bottom) c-kit+ cells treated with PBS vehicle (left panels) or ORY (right panels). (B) Numbers of CD24+ cDCs following differentiation of normal WT or Irf8-KO c-kit+ cells treated with PBS or ORY. 3–5 technical replicates were performed for each sample and averaged together. Horizontal bars represent the mean value and error bars represent standard deviation. p-values were obtained using unpaired t-test.