Figure 5: Model of full-length AR domain organization and its NTD-mediated SRC-3 and p300 coactivator recruitment.
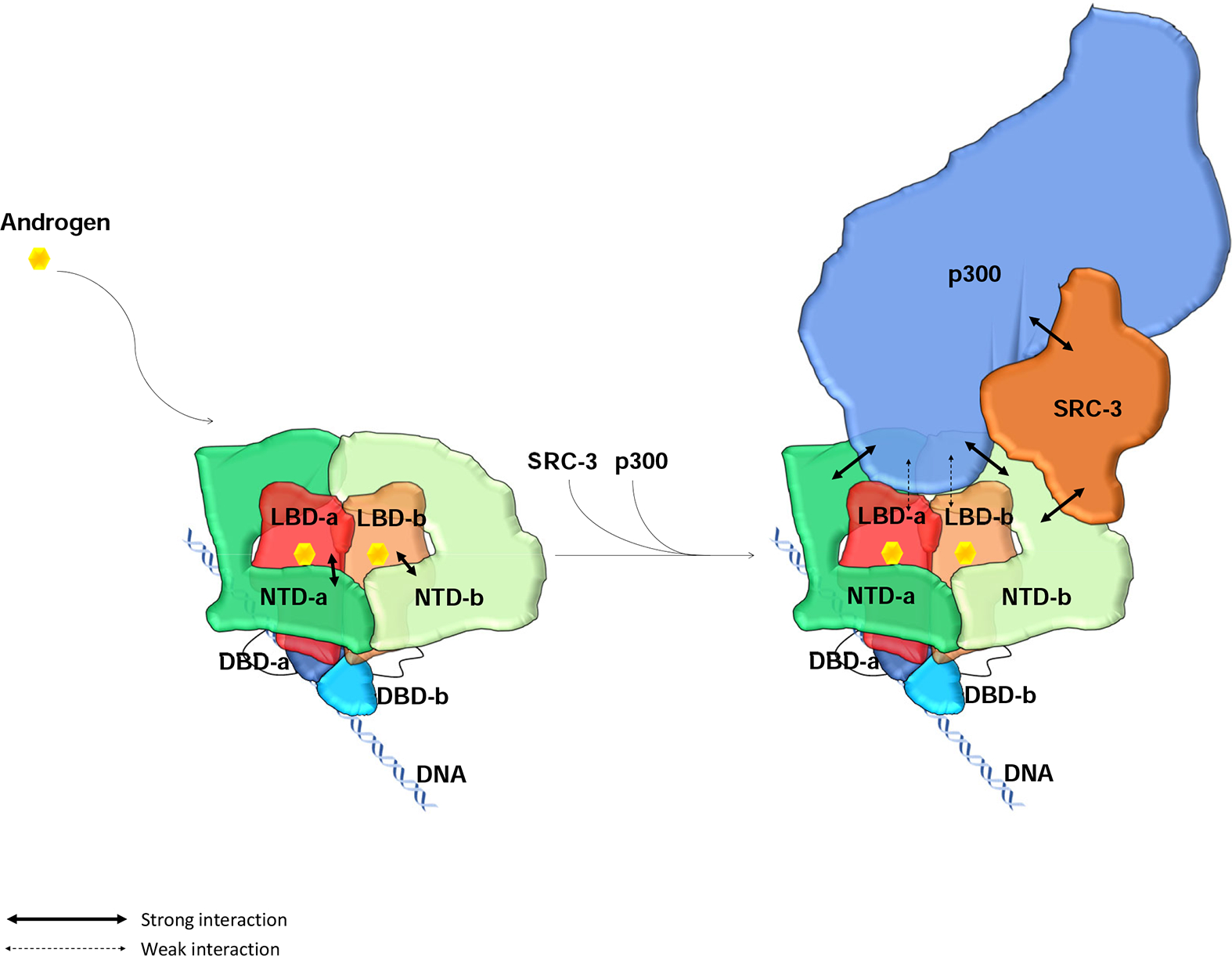
AR dimerizes upon binding to its ligand androgen. Its LBD and DBD are located at the center to form a tight dimerization interface. The two NTDs in the dimer adopt slightly different conformations, wrap around the LBDs, and make tight intra- and inter-molecular N/C interactions. The NTDs also connect to each other to contribute to the dimerization. Through these interactions, AR dimer forms a unique head-to-head and tail-to-tail dimer. AR dimer then recruits key coactivators, SRC-3 and p300, to ARE DNA mainly through its NTDs. One molecule of SRC-3 interacts with one of the AR NTDs (NTD-b) while p300 interacting with both AR NTDs as well as the LBDs to form a stable transcriptionally active complex.
