Abstract
Objective
To evaluate serum estradiol (E2) concentrations during use of 90-day contraceptive vaginal rings releasing E2 75, 100, or 200 mcg/day and segesterone acetate (SA) 200 mcg/day to identify a dose that avoids hypoestrogenism.
Study Design
We conducted a multicenter dose-finding study in healthy, reproductive-aged women with regular cycles with sequential enrollment to increasing E2 dose groups. We evaluated serum E2 concentrations twice weekly for the primary outcome of median E2 concentrations throughout initial 30-day use (target ≥40 pg/mL). In an optional 2-cycle extension substudy, we randomized participants to 2- or 4-day ring-free intervals per 30-day cycle to evaluate bleeding and spotting based on daily diary information.
Results
Sixty-five participants enrolled in E2 75 (n=22), 100 (n=21), and 200 (n=22) mcg/day groups; 35 participated in the substudy. Median serum E2 concentrations in 75 and 100 mcg/day groups were <40 pg/mL. In the 200 mcg/day group, median E2 concentrations peaked on days 4–5 of CVR use at 194 pg/mL (range 114–312 pg/mL) and remained >40 pg/mL throughout 30 days; E2 concentrations were 37 pg/mL (range 28–62 pg/mL) on days 88–90 (n=11). Among the E2 200 mcg/day substudy participants, all had withdrawal bleeding following ring removal. The 2-day ring-free interval group reported zero median unscheduled bleeding and two (range 0–16) and three (range 0–19) unscheduled spotting days in extension cycles 1 and 2, respectively. The 4-day ring-free interval group reported zero median unscheduled bleeding or spotting days.
Conclusions
Estradiol concentrations with rings releasing E2 200 mcg/day and SA 200 mcg/day avoid hypoestrogenism over 30-day use.
Implications
A 90-day contraceptive vaginal ring releasing estradiol 200 mcg/day and segesterone acetate 200 mcg/day achieves estradiol concentrations that should avoid hypoestrogenism and effectively suppresses ovulation.
Keywords: segesterone acetate, Nestorone®, estradiol, clinical trial, contraception, vaginal ring
1.0. Introduction
Segesterone acetate (SA, also known as Nestorone®) is a progestin that has low bioavailability with oral administration, but demonstrates potent activity via vaginal, transdermal, or subdermal routes [1]. While SA-only vaginal rings releasing up to 100 mcg/day demonstrated effective ovulation inhibition with continuous use [2], follicular development persisted, which could result in ovulation with breaks in usage (e.g. a 7-day ring-free interval). In addition, concerns regarding unfavorable bleeding profiles led to addition of estrogen during ring development. Subsequently, a contraceptive vaginal system releasing ethinyl estradiol 13 mcg/day and SA 150 mcg/day for cyclic use over one year has recently received FDA approval, offering women a user-controlled, combined hormonal method with a longer duration of action compared with other available combined hormonal methods [3–5].
Contraceptive products containing ethinyl estradiol may increase the risk of venous thromboembolism, especially among obese women [6–8]. This risk may be increased even when ethinyl estradiol is delivered vaginally due to systemic absorption and significant second-pass hepatic metabolism [9–12]. Alternatively, a contraceptive ring delivering 17-beta estradiol (E2) with a progestin should not increase thromboembolic risk as ethinyl estradiol does [13]. This product may offer safety advantages to users [14], particularly to those who are obese.
As higher doses of SA may lead to hypoestrogenism by preventing ovarian folliculogenesis and estrogen production [2], an adequate E2 replacement dose in the vaginal delivery device is required. A previous Phase 2a dose-finding study evaluated serum E2 concentrations with a 90-day vaginal ring releasing E2 at 10, 20, or 40 mcg/day with SA 200 mcg/day. These rings included a higher SA dose to ensure complete ovarian suppression, including in obese women, given the potentially less gonadotropic effect from E2 as opposed to ethinyl estradiol [14]. However, despite increasing E2 dose release, none of the rings achieved target serum E2 concentrations (≥40 pg/mL) [14,15]. We performed this study to evaluate serum E2 concentrations with use of a contraceptive vaginal ring (CVR) releasing higher E2 doses. In addition, we assessed bleeding patterns and side effects.
2.0. Materials and methods
Seven sites of the NICHD Contraceptive Clinical Trials Network (CCTN) conducted a multicenter, open-label, dose-finding study to evaluate serum E2 concentrations over 30 days with three E2/SA CVRs releasing different E2 doses. The E2/SA CVR is comprised of a silicone elastomer; SA and E2 are mixed with elastomer and extruded to form the ring. The target diameter and cross section of the rings ranged from 56.4 to 56.6 mm and 8.10 to 8.20 mm, respectively. For this study, the Population Council manufactured three dosage formulations with a target SA release rate of 200 mcg/day combined with E2 75, 100 or 200 mcg/day. In vitro testing confirmed the rings released their targeted E2 dose through 90 days.
The sites for this trial included Columbia University, Eastern Virginia Medical School, Oregon Health and Science University, University of California, Davis, University of Cincinnati, University of Pennsylvania, and the University of Utah. The Chesapeake Institutional Review Board (IRB) served as the central site for protocol approval; each site’s local IRB also approved the study and individual participants signed written informed consent.
We used the same entry criteria as the prior dose-finding study with lower E2 doses [14]. Briefly, we enrolled healthy women 18–39 years of age with regular menstrual cycles when not using hormonal contraception, an intact uterus and both ovaries, and who were willing to abstain from non-water based vaginal lubricant use. We excluded women with known hypersensitivity to progestins, estrogen, or silicone rubber, contraindication to combined estrogen-progestin contraceptive use, injectable contraceptive use within nine months prior to enrollment or without a spontaneous menses since last injection, history of toxic shock syndrome, anatomical abnormality that precluded use of a vaginal ring (e.g. cystocele), severe constipation, or body mass index ≥ 35 kg/m2. We also excluded women using isotretinoin, sex steroid hormonal medications, vaginal treatment for other illnesses, or CYP3A4 liver enzyme-inducing or inhibiting medications. Women using hormonal contraception must have discontinued use at least seven days before enrollment.
After the screening visit, participants returned during the first five days of the next spontaneous menses for enrollment. We obtained pre-treatment E2 concentrations prior to ring insertion at this visit. On-treatment visits occurred twice weekly for one month to collect blood samples for E2, SA, and progesterone measurement and to review diary cards to identify ring problems, adverse events (AEs), concomitant treatments, and any ring removals and reinsertions. We assessed spotting and bleeding using a questionnaire administered on a weekly basis. Participants had the option to complete their last visit on day 28–30 of CVR use or to enroll in a two-cycle extension substudy to evaluate bleeding patterns. We randomized those interested in the substudy to initiate either a 2- or 4-day ring-free interval at the end of each 30-day cycle and asked these participants to complete a daily bleeding diary. We followed participants after completion of CVR use until the first spontaneous menses.
We planned to enroll 17–21 participants in sequential dose-escalating groups that received rings releasing E2 doses of 75, 100, or 200 mcg/day. We did not conduct a formal power and sample size calculation; enrollment targets were intended to provide measures of central tendency consistent with a proof of concept Phase 2 dose-finding study.
The primary outcome of this study was median serum E2 concentrations during 30 days of CVR use with a target of ≥40 pg/mL. While the CVRs are designed for use over 90 days, a prior trial with this ring design demonstrated that we can determine whether E2 concentrations would reach our target within the first 30-day period, allowing an earlier assessment of the suitability of these doses for further investigation [14]. Secondary outcomes included treatment compliance (based on SA concentrations ≥ 40 pg/mL), ovulation suppression, bleeding, satisfaction, side effects, and E2 concentrations in a subset of participants evaluated at 90-days of CVR use.
The Endocrine Technologies Core at the Oregon National Primate Research Center measured E2 and SA concentrations using liquid chromatography-tandem mass spectrometry (LC-MS/MS). For E2, the inter-assay and intra-assay precisions were 5.3% and 4.8%, respectively [16]. For SA, the inter-assay and intra-assay precisions were 14.0% and 10.7%, respectively. The lower limit of quantitation for both assays was 10 pg/mL. We defined ovulation based on laboratory criteria of two consecutive progesterone values of ≥3 ng/mL or a single progesterone concentration >10 ng/mL. We determined bleeding satisfaction with questions about bleeding and spotting in the previous seven days during the main study. For bleeding pattern analysis in the extension substudy, we based the scheduled bleeding window on criteria defined by Mishell et al [17] as any bleeding or spotting during the hormone-free interval and through the first 4 days of the next cycle. Because the hormone-free window differed between the two groups, we used an 8-day scheduled bleeding window for analyses of both groups. Unscheduled bleeding or spotting referred to any bleeding or spotting that occurred outside of this window. Safety assessments included AE collection, physical examination, and laboratory evaluation of hematologic, chemistry, and lipid parameters. We performed Fisher’s Exact test and Cochran-Armitage trend test using SAS software.
3.0. Results
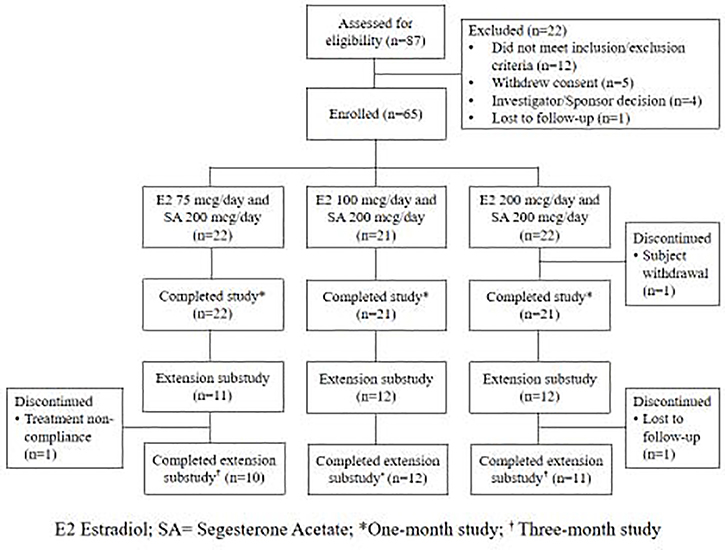
We enrolled 65 women who received CVRs releasing E2 75 mcg/day (n=22), E2 100 mcg/day (n=21), or E2 200 mcg/day (n=22) along with SA 200 mcg/day; 64 of these participants completed the main study. Thirty-five participants opted to continue in the extension substudy, and 33 completed the additional 60 days of CVR use (Figure 1). Participants were primarily non-Hispanic white and not currently using tobacco products; about half were overweight or obese (Table 1).
Figure 1.
Subject participation in the main and extension studies of three contraceptive vaginal rings releasing estradiol and segesterone acetate
Table 1.
Participant characteristics in a dose-finding study of contraceptive vaginal ring releasing estradiol 75 mcg, 100 mcg, or 200 mcg per day and segesterone acetate 200 mcg per day
| E2 75 mcg/day and SA 200 mcg/day n=22 |
E2 100 mcg/day and SA 200 mcg/day n=21 |
E2 200 mcg/day and SA 200 mcg/day n=22 |
|
|---|---|---|---|
| Age (years) | 27.0 ± 5.4 | 27.8 ± 6.6 | 30.0 ± 5.4 |
| Non-Hispanic ethnicity | 19 (86.4) | 20 (95.2) | 21 (95.5) |
| Race | |||
| White | 13 (59.1) | 14 (66.7) | 15 (68.2) |
| Black | 6 (27.3) | 3 (14.3) | 5 (22.7) |
| Other | 3 (13.6) | 4 (19.0) | 2 (9.1) |
| Gravidity | |||
| 0 | 14 (66.7) | 11 (52.4) | 13 (59.1) |
| 1 | 3 (14.3) | 2 (9.5) | 4 (18.2) |
| 2 or more | 4 (18.2) | 8 (38.1) | 5 (22.7) |
| Parity | |||
| 0 | 16 (72.7) | 13 (61.9) | 17 (77.3) |
| 1 | 1 (4.5) | 2 (10.0) | 2 (9.1) |
| 2 or more | 4 (18.2) | 6 (28.6) | 3 (13.6) |
| Weight (kg) | 71.2 ± 13.6 | 67.4 ± 12.7 | 70.8 ± 15.1 |
| Body Mass Index (kg/m2) | 25.9 ± 4.2 | 25.3 ± 4.5 | 25.8 ± 4.8 |
| <18.5 | 0 | 2 (9.5) | 1 (4.5) |
| 18.5 – <25.0 | 10 (45.5) | 9 (42.9) | 12 (54.5) |
| 25.0 – <30.0 | 6 (27.3) | 5 (23.8) | 4 (18.2) |
| ≥30.0 | 6 (27.3) | 5 (23.8) | 5 (22.7) |
| Current tobacco use | 0 | 2 (9.5) | 0 |
Data presented as mean ± standard deviation or n (%)
E2 = estradiol; SA = Segesterone Acetate
The median pre-treatment E2 concentration for all participants was 36.5 pg/mL (range 5–110 pg/mL). Figure 2 presents the E2 concentrations of all treatment groups. Median E2 concentrations remained low (<40 pg/mL) after seven days in those using CVRs releasing E2 75mcg/day and E2 100 mcg/day with marginal dose response. In contrast, median E2 concentrations peaked on treatment days 4 or 5 at 194 pg/mL (range 114–312 pg/mL) with E2 200 mcg/day CVR use and declined to 51.5 pg/mL (range 21–109 pg/mL) at day 30. Median E2 concentration was 37 pg/mL (range 28–62 pg/mL) for the 11 participants using this dose ring on treatment days 88 to 90 in the extension substudy. We identified no ovulations during study participation with any E2/SA dose product. Overall, 21 (95.5%), 20 (95.2%), and 19 (86.4%) of participants using the E2 75 mcg/day, E2 100 mcg/day, and E2 200 mcg/day rings, respectively, demonstrated full compliance with ring use as determined by SA concentrations.
Figure 2.
Median estradiol concentration with use of a contraceptive vaginal ring releasing estradiol 75, 100, or 200 mcg/day and segesterone acetate 200 mcg/day. Serum hormone concentrations were not assayed between day 30 and day 88.
Bleeding and spotting occurred in all users during the first CVR week as women initiated ring use during menses. Participants using the E2 75 and 100 mcg/day CVRs reported more bleeding and spotting (Table 2). In contrast, only two E2 200 mcg/day CVR users reported any bleeding or spotting in weeks 2, 3, and 4 of the initial treatment cycle. Further, 20 of 43 (47%) participants using the E2 75 and 100 mcg/day CVRs considered the bleeding and spotting to be “bothersome” compared with 2 of 22 (9%) participants using the E2 200 mcg/day CVR (p=0.003).
Table 2.
Spotting and bleeding and bothersome assessment in the first month of using a contraceptive vaginal ring releasing estradiol 75 mcg, 100 mcg, or 200 mcg per day and segesterone acetate 200 mcg per day
| E2 75 mcg/day and SA 200 mcg/day n=22 |
E2 100 mcg/day and SA 200 mcg/day n=21 |
E2 200 mcg/day and SA 200 mcg/day n=22 |
|
|---|---|---|---|
| Week 1*ǂ | |||
| Spotting | 7 (31.8) | 3 (14.3) | 6 (27.3) |
| Bothersome | 0 | 1 (33.3) | 2 (33.3) |
| Bleeding | 8 (36.4) | 2 (9.5) | 6 (27.3) |
| Bothersome | 0 | 0 | 3 (50.0) |
| Week 2* | |||
| Spotting | 4 (18.2) | 5 (23.8) | 1 (4.5) |
| Bothersome | 3 (75.0) | 2 (40.0) | 1 (100) |
| Bleeding | 1 (4.5) | 4 (19.0) | 0 |
| Bothersome | 1 (100) | 4 (100) | 0 |
| Week 3* | |||
| Spotting | 7 (31.8) | 5 (23.8) | 0 |
| Bothersome | 5 (71.4) | 4 (80.0) | 0 |
| Bleeding | 4 (18.2) | 4 (19.0) | 0 |
| Bothersome | 4 (100) | 2 (50.0) | 0 |
| Week 4* | |||
| Spotting | 10 (45.5) | 8 (38.1) | 0 |
| Bothersome | 5 (50.0) | 7 (87.5) | 0 |
| Bleeding | 7 (31.8) | 4 (19.0) | 1 (4.5) |
| Bothersome | 5 (71.4) | 2 (50.0) | 1 (100) |
Participants reported bleeding or spotting in week prior. Bothersome assessment done in participants who reported spotting or bleeding.
Participants initiated ring use during menses so the bleeding and spotting in week 1 include residual menses from a cycle prior to treatment.
Data presented as n (%); SA = Segesterone Acetate; E2 = estradiol
In the extension substudy, withdrawal bleeding occurred in all participants following E2 200 mcg/day CVR removal as opposed to the lower dose rings (Table 3). Among E2 200 mcg/day CVR users, those randomized to the 4-day ring-free interval reported a median of 0 unscheduled bleeding or spotting days. Participants randomized to the 2-day ring-free interval reported a median of zero unscheduled bleeding days and a median of two (range 0–16) and three (range 0–19) unscheduled spotting days in the first and second 30-day extension cycles, respectively. Among participants randomized to the 2-day ring-free interval, more women reported spotting with increasing E2 doses (E2 75 mcg/day: 0/5; E2 100 mcg/day: 2/6; E2 200 mcg/day: 4/7; p=0.04).
Table 3.
Scheduled and unscheduled bleeding and spotting with 2-day and 4-day ring-free intervals in participants using a contraceptive vaginal ring releasing estradiol 75 mcg, 100 mcg, or 200 mcg per day and segesterone acetate 200 mcg per day
| Ring dose | n | First 30-day extension | Second 30-day extension | |||||
|---|---|---|---|---|---|---|---|---|
| Scheduled bleeding | Unscheduled bleeding | Unscheduled spotting | Scheduled bleeding | Unscheduled bleeding | Unscheduled spotting | |||
| 2 day ring-free interval | E2 75 mcg/day and SA 200 mcg/day | 5 | 3 (60%) | 0* | 0 (0–11) | 3 (60%) | 0 (0–4) | 0* |
| E2 100 mcg/day and SA 200 mcg/day | 6 | 4 (67%) | 0 (0–7) | 0 (0–15) | 6 (100%) | 0 (0–2) | 0.5 (0–1) | |
| E2 200 mcg/day and SA 200 mcg/day | 7 | 7 (100%) | 0 (0–2) | 2 (0–16) | 7 (100%) | 0* | 3 (0–19) | |
| 4 day ring-free interval | E2 75 mcg/day and SA 200 mcg/day | 6 | 6 (100%) | 0 (0–3) | 0.5 (0–12) | 6 (100%) | 0 (0–4) | 2.5 (0–6) |
| E2 100 mcg/day and SA 200 mcg/day | 6 | 4 (67%) | 0* | 0.5 (0–6) | 6 (100%) | 0 (0–17) | 0 (0–1) | |
| E2 200 mcg/day and SA 200 mcg/day | 4 | 4 (100%) | 0* | 0 (0–4) | 4 (100%) | 0* | 0 (0–2) | |
Data presented as n (%) or median (range).
SA= Segesterone Acetate; E2= Estradiol
No range presented because all participants reported no unscheduled bleeding or spotting
Most participants reported at least one AE during the treatment period with the most frequently reported AEs being headaches and breast tenderness (Table 4). No early discontinuations occurred due to an AE. An investigator reported one serious AE of gastritis unrelated to the study drug.
Table 4.
Adverse events with contraceptive vaginal ring use releasing estradiol 75 mcg, 100 mcg, or 200 mcg per day and segesterone acetate 200 mcg per day*
| E2 75 mcg/day and SA 200 mcg/day n=22 |
E2 100 mcg/day and SA 200 mcg/day n=21 |
E2 200 mcg/day and SA 200 mcg/day n=22 |
|
|---|---|---|---|
| Total number of participants with at least one AE | 18 (81.8) | 12 (57.1) | 16 (72.7) |
| Headache | 8 (36.4) | 4 (19.0) | 5 (22.7) |
| Breast tenderness | 1 (4.5) | 0 | 5 (22.7) |
| Urinary tract infection | 2 (9.1) | 0 | 3 (13.6) |
| Dysmenorrhea | 2 (9.1) | 1 (4.8) | 2 (9.1) |
| Nausea | 2 (9.1) | 1 (4.8) | 2 (9.1) |
| Dizziness | 2 (9.1) | 2 (9.5) | 0 |
| Nasopharyngitis | 2 (9.1) | 1 (4.8) | 0 |
| Vaginal odor | 2 (9.1) | 1 (4.8) | 0 |
| Vulvovaginal mycotic infection | 2 (9.1) | 1 (4.8) | 0 |
| Urinary frequency | 1 (4.5) | 0 | 2 (9.1) |
| Affect lability | 0 | 2 (9.5) | 1 (4.5) |
| Abdominal distension | 0 | 0 | 3 (13.6) |
Only AEs experienced by 3 or more study participants were included in this table
E2 = estradiol; SA = Segesterone acetate; AE = adverse event
4.0. Discussion
We evaluated three E2/SA vaginal rings with different E2 release rates in this dose-finding study to identify the lowest dose that would meet a predefined endpoint of median E2 serum concentrations above 40 pg/mL. We found that only the CVR delivering the highest E2 dose (E2 200 mcg/day) met the study goal. Further, this ring appeared to effectively suppress ovulation without significant side effects. Based on these findings, we chose the CVR releasing E2 200 mcg/day and SA 200 mcg/day for a Phase IIb study to evaluate contraceptive efficacy during one year of cyclic or continuous use (NCT03432416).
The lowest E2 dose ring (75 mcg/day) yielded E2 concentrations similar to the previous dose-finding study [14]. We found a marginal E2 dose-response with the E2 100 mcg/day dose, but the median serum levels remained below the target. Median serum E2 concentrations increased above 40 pg/mL only with E2 200 mcg/day CVR, suggesting that a threshold dose of E2 released from the CVR is needed to provide adequate sustainable E2 concentrations that could prevent hypoestrogenism resulting from suppression of the hypothalamic-pituitary-ovarian axis by SA.
We acknowledge limitations with setting our study goal of achieving E2 concentrations of ≥ 40 pg/mL given long-term health implications of hypoestrogenism, including bone health. Previous studies with depot medroxyprogesterone acetate (DMPA) raised concern about peak bone mass in young users and demonstrated adverse bone mineral density changes with low serum estradiol concentrations [18–20]. With the E2 200 mcg/day ring, we found median serum E2 concentrations >50 pg/mL in the first 30 days of ring use before decreasing to 37 pg/mL at the end of the 90-day treatment period. Placement of a new ring at that point should lead to recovery of serum E2 concentrations and avoid sustained low E2 concentrations. Future proposed evaluations include measurements of E2 concentrations with sequential ring use and bone health to confirm that serum E2 concentrations with this product are sufficient for avoiding hypoestrogenism.
Contraceptives using E2 rather than ethinyl estradiol have generally resulted in bleeding patterns with high rates of unscheduled bleeding. The first E2-containing pill (estradiol valerate and dienogest) featured a complex quadriphasic regimen to improve cycle control [21]. With the second combined hormonal pill containing E2 and nomegestrol acetate in a 24/4 regimen, women experienced a decrease in unscheduled bleeding over time and increase in amenorrhea with continued use [22]. Bleeding patterns with investigational CVRs containing E2 and various doses of nomegestrol or etonogestrel showed that unscheduled bleeding decreases over time with all rings; however, bleeding predictability may not compare with ethinyl estradiol-based contraceptives [23, 24]. In this study, we evaluated CVRs containing three doses of E2 combined with a progestin dose that effectively suppresses follicular development and endogenous ovarian estrogen synthesis. Women using the E2 200 mcg/day CVR reported fewer bothersome unscheduled bleeding or spotting days in the first 30 days of use compared with women using lower E2 dose CVRs. This initial experience may be a critical time for determining acceptability of the method for new users. Additionally, cyclic ring-free intervals appeared to result in predictable withdrawal bleeds for most participants. Among participants using the E2 200 mcg/day CVR, the only dose that met target serum E2 concentrations, less unscheduled bleeding or spotting occurred in the group assigned to a 4-day ring-free interval group compared to the group assigned to a 2-day ring-free interval. However, the small number of participants in the extension substudy and the short data collection period (i.e. two 30-day cycles) limit our ability to predict the bleeding patterns in a larger group of women.
We have determined that the CVR releasing E2 200 mcg/day and SA 200 mcg/day is capable of achieving adequate E2 concentrations to avoid hypoestrogenism. Additional evaluations are currently underway to determine contraceptive efficacy, bleeding patterns, safety, and acceptability of this product.
Acknowledgements
The authors would like to acknowledge Sarah Godfrey and Clint Dart at Health Decisions, Drs. Alicia Christy and Mark Payson at the National Institutes of Health, and Dr. Ruth Merkatz at Population Council.
The vaginal rings were engineered under a proprietary method and manufactured in the GMP laboratory of the Population Council, New York, holder of the IND 114,380.
The Endocrine Technologies Core (ETC) at the Oregon National Primate Research Center (ONPRC) is supported by NIH Grant P51 OD011092 awarded to ONPRC.
Funding
The study was supported by NICHD Contraception Clinical Trial Network: HHSN275201300010I/HHSN27500003 (Columbia University),
HHSN275201300021I/HHSN27500002 (UC Davis), HHSN275201300014I/HHSN27500003 (University of Cincinnati), HHSN275201300020I/HHSN27500003 (University of Pennsylvania), HHSN275201300016I/HHSN27500002 (University of Utah), HHSN275201300019I/HHSN27500003 (Eastern Virginia Medical School), HHSN275201300008I/HHSN27500005 (Oregon Health and Science University). MJC was additionally supported by NICHD K23 HD090323.
Disclosures:
Dr. Creinin serves on an Advisory Board for Lupin and Merck and is a consultant for Danco, Estetra SPRL, Exeltis, and Medicines360. The Department of Obstetrics and Gynecology, University of California, Davis, receives research funding for contraceptive research from Daré, HRA Pharma, Medicines360, and Sebela Pharmaceuticals.
The Department of Obstetrics and Gynecology, Division of Family Planning at the University of Utah receives research funding from Bayer, Cooper Surgical, Medicines360, Merck, and Sebela Pharmaceuticals. Dr. Turok is a consultant for Sebela Pharmaceuticals.
Dr. Archer has received consulting fees from AbbVie, Agile Therapeutics, Exeltis, Endoceutics, ObsEva, Radius and TherapeuticsMD. Eastern Virginia Medical School has received research support from Abbvie, Bayer Healthcare, Exeltis, Endoceutics, Myovant, ObsEva, Radius, and TherapeuticsMD.
Dr. Westhoff serves as a consultant for Merck and Bayer as a DSMB member for several phase 4 studies, consults for AbbVie, and receives research funding from Medicines360 and Sebela Pharmaceuticals.
The Department of Obstetrics and Gynecology at the University of Cincinnati College of Medicine accepts research monies from Medicines360, Bayer Healthcare, and Sebela Pharmaceuticals.
Dr. Jensen has received payments for consulting from Abbvie, Cooper Surgical, Bayer Healthcare, Merck, Sebela Pharmaceuticals, and the Population Council. OHSU has received research support from Abbie, Bayer Healthcare, Daré, Estetra SPRL, Medicines360, Merck, and Sebela Pharmaceuticals. These companies and organizations may have a commercial or financial interest in the results of this research and technology. These potential conflicts of interest have been reviewed and managed by OHSU.
Dr. Sitruk-Ware and Dr. Variano are employees of the Population Council, a not for profit organization, IND holder for Nestorone formulations, and developer of the vaginal ring described in this paper.
Dr. Blithe and Dr. Long are employees of the NIH.
Footnotes
Dr. Chen and Dr. Barnhart have no financial conflicts of interest to disclose.
ClinicalTrials.gov Identifier: NCT02626208
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sitruk-Ware R, Small M, Kumar N, Tsong YY, Sundaram K, Jackanicz T. Nestorone®: clinical applications for contraception and HRT. Steroids 2003;68:907–13. [DOI] [PubMed] [Google Scholar]
- [2].Brache V, Mishell DR, Lahteenmaki P, Alvarez F, Elomaa K, Jackanicz T, et al. Ovarian function during use of vaginal rings delivering three different doses of Nestorone. Contraception 2001;63:257–61. [DOI] [PubMed] [Google Scholar]
- [3].Archer DF, Merkatz RB, Bahamondes L, Westhoff CL, Darney P, Apter D, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health 2019;7:e1054–e1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, Thomas M, Barnhart KT, Creasy G, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception 2019;99:323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Merkatz RB, Plagianos M, Hoskin E, Cooney M, Hewett PC, Mensch BS. Acceptability of the Nestorone®/ethinyl estradiol contraceptive vaginal ring: development of a model; implications for introduction. Contraception 2014;90:514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Peragallo Urrutia R, Coeytaux RR, McBroom AJ, Gierisch JM, Havrilesky LJ, Moorman PG, et al. Risk of acute thromboembolic events with oral contraceptive use: a systematic review and meta-analysis. Obstet Gynecol 2013;122:380–9. [DOI] [PubMed] [Google Scholar]
- [7].Sitruk-Ware R Hormonal contraception and thrombosis. Fertil Steril 2016;106:1289–94. [DOI] [PubMed] [Google Scholar]
- [8].Horton LG, Simmons KB, Curtis KM. Combined hormonal contraceptive use among obese women and risk for cardiovascular events: a systematic review. Contraception 2016;94:590–604. [DOI] [PubMed] [Google Scholar]
- [9].Archer DF, Thomas MA, Conard J, Merkatz RB, Creasy GW, Roberts K, et al. Impact on hepatic estrogen-sensitive proteins by a 1-year contraceptive vaginal ring delivering Nestorone® and ethinyl estradiol. Contraception 2016;93:58–64. [DOI] [PubMed] [Google Scholar]
- [10].Sitruk-Ware R, Plu-Bureau G, Menard J, Conard J, Kumar S, Thalabard JC, et al. Effects of oral and transvaginal ethinyl estradiol on hemostatic factors and hepatic proteins in a randomized, crossover study. J Clin Endocrinol Metab 2007;92:2074–9. [DOI] [PubMed] [Google Scholar]
- [11].Lidegaard O, Nielsen LH, Skovlund CW, Lokkegaard E. Venous thrombosis in users of non-oral hormonal contraception: follow-up study, Denmark 2001–10. BMJ 2012;344:e2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tepper NK, Dragoman MV, Gaffield ME, Curtis KM. Nonoral combined hormonal contraceptives and thromboembolism: a systematic review. Contraception 2017;95:130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dinger J, Do Minh T, Heinemann K. Impact of estrogen type on cardiovascular safety of combined oral contraceptives. Contraception 2016;94:328–39. [DOI] [PubMed] [Google Scholar]
- [14].Jensen JT, Edelman AB, Chen BA, Archer DF, Barnhart KT, Thomas MA, et al. Continuous dosing of a novel contraceptive vaginal ring releasing Nestorone® and estradiol: pharmacokinetics from a dose-finding study. Contraception 2018;97:422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tiedeken M, Westhoff CL, Cohen A, Cremers S, Sitruk-Ware R, Blithe DL; NICHD Contraceptive Trials Network Vaginal Ring Group. Bone turnover markers in women participating in a dose-finding trial of a contraceptive vaginal ring releasing Nestorone and estradiol. Contraception 2019;99:329–34. [DOI] [PubMed] [Google Scholar]
- [16].Blue SW, Winchell AJ, Kaucher AV, Lieberman RA, Gilles CT, Pyra MN, et al. Simultaneous quantitation of multiple contraceptive hormones in human serum by LC- MS/MS. Contraception 2018;97:363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mishell DR Jr, Guillebaud J, Westhoff C, Nelson AL, Kaunitz AM, Trussell J, et al. Recommendations for standardization of data collection and analysis of bleeding in combined hormone contraceptive trials. Contraception 2007;75:11–5. [DOI] [PubMed] [Google Scholar]
- [18].Clark MK, Sowers M, Nichols S, Levy B. Bone mineral density changes over two years in first-time users of depot medroxyprogesterone acetate. Fertil Steril 2004;82:1580–6. [DOI] [PubMed] [Google Scholar]
- [19].Clark MK, Sowers M, Levy BT, Tenhundfeld P. Magnitude and variability of sequential estradiol and progesterone concentrations in women using depot medroxyprogesterone acetate for contraception. Fertil Steril 2001;75:871–7. [DOI] [PubMed] [Google Scholar]
- [20].Kaunitz AM, Darney PD, Ross D, Wolter KD, Speroff L. Subcutaneous DMPA vs intramuscular DMPA: a 2-year randomized study of contraceptive efficacy and bone mineral density. Contraception 2009;80:7–17. [DOI] [PubMed] [Google Scholar]
- [21].Ahrendt HJ, Makalova D, Parke S, Mellinger U, Mansour D. Bleeding pattern and cycle control with an estradiol-based oral contraceptive: a seven-cycle randomized comparative trial of estradiol valerate/dienogest and ethinyl estradiol/levonorgestrel. Contraception 2009;80:436–44. [DOI] [PubMed] [Google Scholar]
- [22].Mansour D, Westhoff C, Kher U, Korver T. Pooled analysis of two randomized, open-label studies comparing the effects of nomegestrol acetate/17β -estradiol and drospirenone/ethinyl estradiol on bleeding patterns in healthy women. Contraception 2017;95:390–7. [DOI] [PubMed] [Google Scholar]
- [23].Duijkers I, Klipping C, Heger-Mahn D, Fayad GN, Frenkl TL, Cruz SM, et al. Phase II dose-finding study on ovulation inhibition and cycle control associated with the use of contraceptive vaginal rings containing 17βestradiol and the progestogens etonogestrel or nomegestrol acetate compared to NuvaRing. Eur J Contracept Reprod Health Care 2018;23:245–54. [DOI] [PubMed] [Google Scholar]
- [24].Vieira CS, Fraser IS, Plagianos MG, Burke AE, Westhoff CL, Jensen J, et al. Bleeding profile associated with 1-year use of the segesterone acetate/ethinyl estradiol contraceptive vaginal system: pooled analysis from Phase 3 trials. Contraception 2019;100:438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]