Abstract
Introduction:
Despite the safety and efficacy of the human papillomavirus vaccine, thousands are impacted by human papillomavirus and its related cancers. Rural regions have disproportionately low rates of human papillomavirus vaccination. Primary care clinics play an important role in delivering the human papillomavirus vaccine. A positive deviance approach is used to identify workflows, organizational factors, and communication strategies in rural clinics with higher human papillomavirus vaccine up-to-date rates. Positive deviance is a process by which exceptional behaviors and strategies are identified to understand factors that enable success.
Methods:
Rural primary care clinics were rank ordered by human papillomavirus vaccine up-to-date rates using 2018 Oregon Immunization Program data, then recruited via purposive sampling of clinics in the top and bottom quartiles. Two study team members conducted pre-visit interviews, intake surveys, and 2-day observation visits with 12 clinics and prepared detailed field notes. Data were collected October–December 2018 and analyzed using a thematic approach January–April 2019.
Results:
Four themes distinguished rural clinics with higher human papillomavirus vaccine up-to-date rates from those with lower rates. First, they implemented standardized workflows to identify patients due for the vaccine and had vaccine administration protocols. Second, they designated and supported a vaccine champion. Third, clinical staff in higher performing sites were comfortable providing immunizations regardless of visit type. Finally, they used clear, persuasive language to recommended or educated parents/patients about the vaccine’s importance.
Conclusions:
Positive deviance identified characteristics associated with higher human papillomavirus vaccine up-to-date rates in rural primary care clinics. These findings provide guidance for rural clinics to inform human papillomavirus vaccination quality improvement interventions.
INTRODUCTION
The first vaccine for the human papilloma virus (HPV) was licensed in June 2006.1 Since its introduction 13 years ago, the vaccine has demonstrated its safety and efficacy in preventing HPV and HPV-related cancers.2–4 Despite these successes, approximately 14 million Americans become infected with HPV annually.5 Though the rate of HPV vaccination among adolescents has been on the rise, the up-to-date (UTD) rate in the U.S. was only 49% in 2017.6 The rural adolescent UTD rate significantly lags behind the urban rate by 12% on average,6–8 and by 12.5% in Oregon.9 An adolescent is considered UTD if they are aged 13–17 years and have either: (1) received three doses of the HPV vaccine or (2) received two doses at least 6 months apart with the first dose administered before their 15th birthday.10
Prior research has shown multilevel factors are associated with the rural–urban HPV disparity in adolescent vaccinations. At the patient level, rural residents are less likely to have heard of HPV or the HPV vaccine.7,11,12 Rural patients are also more likely to experience unique challenges, including transportation and access, negative parental attitudes, misinformation, and economic issues.11,13–15 At the clinic level, rural clinicians and clinical staff face barriers related to staffing shortages, insufficient HPV vaccine inventory, time constraints, infrequency of adolescent visits, and discomfort or lack of training regarding effective communication about the vaccine to patients.14,16–18
Regardless of HPV vaccine barriers in rural regions, a subset of rural primary care clinics succeeds in surpassing the national HPV UTD average of 49%. However, little is known about why these clinics are succeeding.6 Findings from surveys and interviews exploring perceived facilitators of rural HPV vaccination rates suggest better communication from healthcare providers, stories from peers, increased access, and cancer prevention framing as potential reasons.19,20 Although interventions to increase HPV vaccine uptake are known,21 there is a notable absence of research that reports on promising HPV vaccine practices in primary care—and in rural settings specifically. Moreover, limited research uses observational methods to describe how higher performing rural primary care clinics overcome barriers to HPV vaccination and thus exceed the vaccination rates of other rural as well as urban clinics. These outliers deviate in a positive direction22–26 and provide opportunities to understand what they are doing to outperform their counterparts who face the same barriers to high rates of vaccination in rural communities. The positive deviance framework, described in detail in the methods, has been in use since the 1970s in public health research and evaluation studies as a way of understanding unusual but effective approaches to improving health in communities by leveraging innovative approaches.22,27
Therefore, this study aims to identify the organizational structures and clinical workflows that enable rural, high-performing primary care clinics to support HPV vaccine delivery. The hypothesis is that these clinics would display more standardization across workflows, have dedicated staff focused on vaccinations, and utilize influential messaging to support HPV vaccinations. Observational methods are used to identify contributory factors, which enabled examination of what contributes to higher HPV vaccination rates—an advantage over survey and self-report methods.18,28–30 A set of recommendations are provided to inform the implementation strategies that practice facilitators31 may use to increase HPV immunization rates in rural primary care settings.
METHODS
This sequential explanatory mixed methods study,32–35 informed by the positive deviance framework,26 was conducted by a multidisciplinary team with expertise in primary care, HPV vaccination, qualitative and mixed methods, and practice transformation. The five steps of the positive deviance framework include: (1) identifying clinics demonstrating higher performance, (2) conducting an in-depth qualitative analyses to generate theories, (3) testing theories in larger samples, and (4) extensively disseminating best practices. The first two steps in the positive deviance framework are reported in this manuscript.
The IRB at Oregon Health & Science University approved this study on September 11, 2018 (IRB# 18660) and verbal consent was obtained from clinic staff. This work was conducted to inform the larger Rural Adolescent Vaccine Enterprise stepped-wedge cluster randomized trial, the protocol of which is detailed elsewhere.36
Study Sample
The sample for this study included primary care clinics in rural Oregon in the highest and lowest quartiles for HPV UTD rates. First, the authors used Oregon Immunization Program’s ALERT Immunization Information System (IIS)37 2018 data (HPV UTD among those aged 13–17 years) to identify primary care clinics in rural Oregon that met the following inclusion criteria: participants in the Vaccines for Children program and located in a rural setting as defined by Rural–Urban Commuting Area Codes (>4)38 or designated as such by the Oregon Office of Rural Health.39 Oregon’s ALERT Immunization Information System (IIS) is a computerized statewide immunization registry that combines data from both the public and private healthcare sectors into one complete record for individuals in Oregon. Denominator and weighting information were described by Robison.40 Rural definitions by Rural–Urban Commuting Area Code and Oregon Office of Rural Health captured a larger number of clinics outside urban and suburban regions. Second, very small clinics (fewer than ten patients aged 11–12 years or <20 patients aged 13–17 years) were excluded to increase the likelihood of seeing of salient encounters during the observation visit. Third, clinics meeting eligibility criteria were rank ordered from lowest to highest HPV UTD rates (range: 8%–75%) and segmented clinics by quartiles. Clinics within highest and lowest quartiles were purposely recruited to participate in study activities based on clinic type, ownership, and geographic region. Higher performers were oversampled at a 2:1 ratio. Based on the hypothesis that high-performing clinics had greater heterogeneity in their immunization approaches, the authors oversampled these clinics to ensure the identification of organizational structures and workflows that could be used to inform quality improvement targets.
Measures
Two members of the five-person study team (RG, LF, IS, CD, MMD) collected data from each of the 12 participating clinics during October–December 2018. Details about steps and purpose of data collection activities are outlined in Figure 1; study instruments are provided in Appendix A. First, team members conducted 45-minute pre-visit phone interviews with a key point of contact at each clinic to build rapport, gain initial insight into vaccine workflows, and finalize timing for the observation. Second, team members distributed a survey to the clinic point of contact within 1 week of the interview and collected this survey prior to the observation. Finally, two team members conducted 2-day observation visits in the 12 participating clinics within a month of the pre-visit interview. During the observations, two study team members simultaneously observed all points of clinical care—from patient check in, to rooming and care provision, to check out. Exam room shadowing occurred only when patients expressly provided verbal consent. Informal conversations with clinic staff were conducted during observation visits in order to clarify key details; these conversations were captured in the resulting observational field notes.
Figure 1.
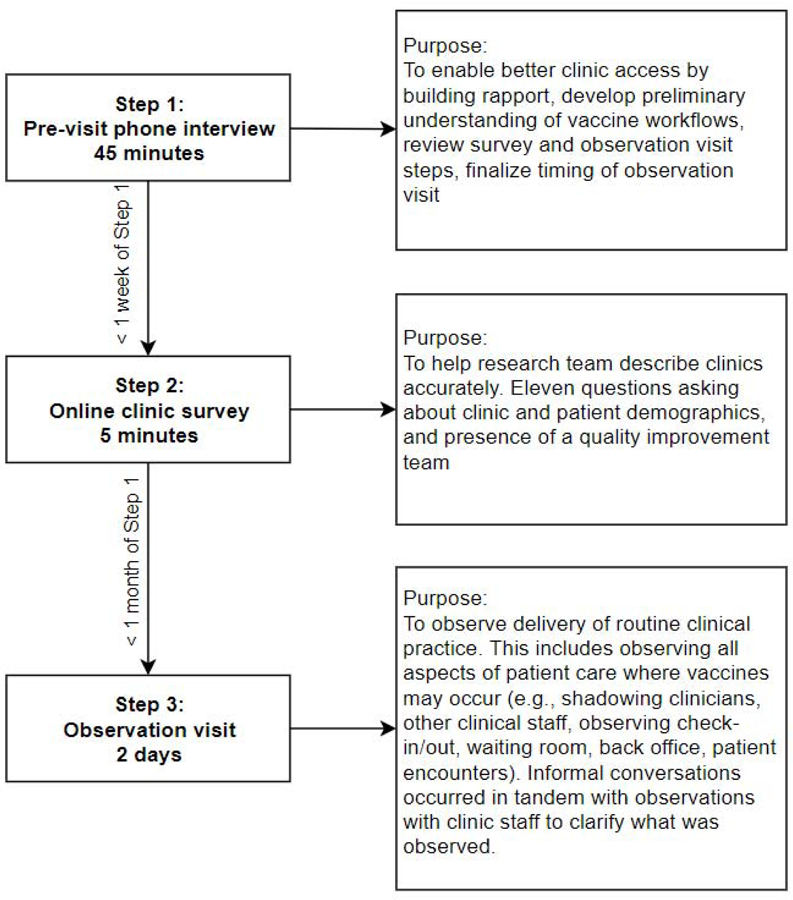
Data collection steps and timing.
Analysis
Data from the pre-visit interviews were used to prepare for the observational visit. Survey data were analyzed using descriptive statistics and used to confirm clinic sampling characteristics. Detailed field notes were prepared following each observation visit within 24 hours and data were transferred to ATLAS.ti, version 8 for data management and analysis. Qualitative data analysis occurred between January and April 2019.
The qualitative team (RG, MMD, LF, IS, CD, AW, JG) utilized a thematic analytic approach, which included immersion in the data, generation of codes, identification, refinement, selection of themes, and writing a detailed analyses.41 The team created a preliminary code list using categories from the Centers for Disease Control and Prevention’s Immunization Quality Improvement Program tool (formerly AFIX).42 The team then refined these a priori codes and added additional inductive codes as the team read field notes aloud and discussed passages during weekly group coding sessions. Once inductive codes stopped being created and agreement was consistently reached by the team on code usage during group coding sessions, field notes were assigned to individual team members for coding. This was also the point at which the team determined data saturation had been reached, as no additional codes related to organizational structure or workflows had emerged.43 The qualitative team prioritized codes for thematic analysis and assigned individual team members a subset of codes to query and analyze. This qualitative team met weekly to share findings, ask questions, and exchange detailed analytic summaries. During this process, two study team members (RG, LF) created a table identifying clinical workflows and behaviors related to HPV vaccination practices and documented their presence or absence in clinics with higher HPV UTD rates. This table and the emerging themes were reviewed monthly with the full Rural Adolescent Vaccine Enterprise study team for discussion and refinement. These forms of peer debriefing and peer review improve validity and rigor in qualitative research.44–47
RESULTS
Performance, ownership, and key characteristics of the 12 participating clinics are described in Table 1. Clinics ranged in size from 70 to 850 patients (of all ages) seen per week and represented rural (n=8) and frontier geographies (n=4). Of the eight higher performing clinics, five were pediatric clinics and three were family medicine clinics. The higher performing clinics had HPV UTD rates between 50% and 70% among patients aged 13–17 years, which are above the national average HPV UTD rate for this age group. The four lower performing clinics were all family medicine clinics and had HPV UTD rates between 13% and 28%. Higher and lower performing clinics used a variety of electronic health record (EHR) platforms (Table 1).
Table 1.
Clinic Characteristics
| Clinic # | HPV UTD rate - 13‒17 year olds (%) | County-level HPV UTD rate - 13‒17 year olds (%) | County population - 10‒19 year olds | Practice type (Ped/FM) | Average patients seen per week (all ages) | Ratio of clinician to clinical staff involved in adolescent immunizations | RUCA code | Office of Rural Health designation | Electronic health record |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 70 | 49 | 4,466 | Ped | 550 | 1:2 | 4 | Frontier | Office practicum |
| 2 | 64 | 46 | 3,255 | Ped | 170 | 1:3 | 4 | Rural | Epic |
| 3 | 60 | 46 | 14,996 | FM | 500 | 1:2 | 4.1 | Rural | Epic |
| 4 | 58 | 42 | 8,784 | Ped | 110 | 1:2 | 4 | Rural | Greenway Prime Suite |
| 5 | 57 | 40 | 677 | FM | 350 | 1:3 | 10 | Frontier | Epic |
| 6 | 53 | 45 | 6,914 | FM | 850 | 1:1 | 2 | Rural | Epic |
| 7 | 50 | 41 | 11,154 | Ped | 260 | 1:2 | 4 | Rural | Greenway Prime Suite |
| 8 | 50 | 45 | 46,336 | Ped | 412 | 1:3 | 4.1 | Rural | NextGen |
| 9 | 28 | 40 | 4,557 | FM | 150 | 1:2 | 4 | Rural | Epic |
| 10 | 18 | 23 | 871 | FM | 270 | 1:1 | 10 | Frontier | EMD |
| 11 | 16 | 22 | 2,156 | FM | 175 | 1:1 | 7 | Rural | AllScripts |
| 12 | 13 | 40 | 677 | FM | 70 | 1:1 | 10 | Frontier | Epic |
HPV, human papilloma virus; UTD, up-to-date; Ped/FM, pediatric/family medicine; RUCA, rural-urban commuting area.
Table 2 indicates key organizational structures and workflow activities identified during the site visit as being associated with HPV vaccine administration in clinics with higher HPV UTD rates. The higher performing clinics shared many similar structures and workflows (Table 2). Four predominant themes emerged that distinguished higher performing clinics’ approaches to reach HPV vaccine goals compared to lower performing clinics. These themes included: (1) staffing and vaccine protocols, (2) presence of a vaccine champion, (3) utilizing all opportunities to vaccinate, and (4) patient communication and education.
Table 2.
Organizational Structures and Key Workflow Activities
| Clinic # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HPV UTD (%) | 70 | 64 | 60 | 58 | 57 | 53 | 50 | 50 | 28 | 18 | 16 | 13 |
| Identifying patients due | ||||||||||||
| Immunization record systematically checked ahead of patient visit | X | X | X | X | X | X | X | |||||
| Delivering vaccine and documentation in EHR and ALERT IIS | ||||||||||||
| Immunizations administered at every visit, if due | X | X | X | X | X | X | ||||||
| Adequate vaccine supply, monitored at least 2x/month | X | X | X | X | ||||||||
| Reminders, recall, and scheduling | ||||||||||||
| Reminder/recall process in place for annual adolescent well visits | X | X | X | X | X | X | ||||||
| System in place to schedule 2nd dose | X | X | ||||||||||
| Access | ||||||||||||
| Follow-up protocol in place for patients who miss an appointment | X | X | X | X | X | |||||||
| Patient communication around vaccine efficacy and safety | ||||||||||||
| HPV vaccine introduced to patient as a part of routine care in a systematic way; Strong recommendation by provider or clinical staff | X | X | X | X | X | X | ||||||
| HPV materials displayed in clinic or handed out to patients, beyond VIS | X | X | X | X | ||||||||
| Performance feedback and staffing | ||||||||||||
| Adolescent immunization rates measured and shared with staff | X | X | X | X | X | |||||||
| Presence of a vaccine champion | X | X | X | X | X | X | X | X | ||||
| Protocol or training in place to deliver the HPV vaccine | X | X | X | X | X | |||||||
| Total | 8 | 10 | 6 | 6 | 10 | 5 | 8 | 6 | 0 | 0 | 0 | 0 |
HPV, human papilloma virus; UTD, up-to-date; EHR, electronic health record; ALERT IIS, ALERT Immunization Information System; VIS, Vaccine Information Statement.
Clinics with higher HPV UTD rates had, on average, additional clinical staff monitoring of immunization workflow irrespective of clinic size. Efforts focusing on vaccine readiness and delivery was most often carried by medical assistants, registered nurses, and clinical staff other than clinicians. In addition to confirming vaccines due and administering them in the clinic, staff worked to monitor vaccine inventory, document and respond to vaccine refrigerator temperatures, and document accurate vaccine information, including lot numbers, in the EHR or ALERT IIS. This burden was mitigated by clinics with EHRs allowing bi-directional data exchange with ALERT IIS, but only three clinic EHRs had this function. Another systems-related factor apparent in these clinics was the standardization of behaviors across all clinical staff. Higher performing clinics had protocols for vaccine administration. This systematized approach to delivering the HPV vaccine coupled with dedicated staff time was woven throughout their organizational activities (Table 2).
All clinics had an assigned Primary Vaccine Coordinator, a federal requirement for those participating in the Vaccines for Children Program. However, the majority of higher performing clinics also had a vaccine champion who not only attended to proper handling procedures and documentation, but also demonstrated a commitment to vaccine administration while promoting a pro-vaccine culture in the clinic. Vaccine champions were clinicians, medicals assistants, and nursing staff. The research team observed the data feedback and monitoring actions of a vaccine champion in the example below:
LPN-1 begins her morning ritual of reconciling inventory. If she sees that a vaccine was missed and there is no note in the EHR, she sends the medical assistant or nurse a note letting them know that they this was a missed opportunity. [Clinic 1]
The level of data monitoring and feedback from this particular vaccine champion (from the above quote) exceeded what was observed in lower performing clinics.
Clinics with lower HPV UTD rates chose to offer the vaccine at adolescent well visits only, which led to missed vaccination opportunities. By contrast, higher performing clinics recommended the vaccine across all visit types (e.g., well, acute, walk-in). All higher performing clinics used this approach, with the exception of Clinic 8 (50% UTD) and Clinic 6 (53% UTD), which did so in a non-systematic fashion. The following example exhibits the spirit of catching all opportunities to provide the HPV vaccine:
NP-1 says that no matter what a person comes in for, they check if they can give vaccines. NP-1 says she doesn’t think they’re doing anything special here at the clinic, they just try to get as many shots in as possible and she just treats vaccines [including HPV] as no big deal, just a regular part of preventive care. NP-1 reiterates that they take every opportunity to vaccinate including if a whole family comes in they will try to vaccinate everyone there at that time. [Clinic 3]
Care team members at higher performing clinics actively communicated information about the HPV vaccine with patients and their guardians in a straightforward manner. These clinic staff communicated approaching due dates for the vaccine and educated patients and guardians about starting the series early. This leveraged the immunization schedule as a tactic to encourage patients to vaccinate at younger ages so they would only need two shots as opposed to three. Various clinic staff played a role in communicating with or educating patients about the HPV vaccine (e.g., front desk staff, medical assistants, registered nurses, clinicians); clinic staff messaged the HPV vaccine in the same manner as other vaccines. Clinic staff made strong recommendations about vaccinations and approached these conversations with confidence and consistency, as demonstrated by the following quote:
NP-12 says she takes a non-judgmental, educational approach when talking about this vaccine with parents. She is trying to sway the message from sex to cancer prevention and likes to be upfront about how boys need it too. NP-1 says that 1% of the population will never get vaccinated, 70% will always get vaccinated, and 29% can be persuaded. NP-1 says going into the discussion with confidence as a provider is the most important aspect to how the results play out. [Clinic 5]
Even in higher performing clinics, there was intermittent outdated messaging related to HPV and the purpose of the vaccine, particularly gendered discrepancies focusing on preventing HPV in women and heteronormative messaging for boys on “protecting your future wife.”
DISCUSSION
This explanatory mixed methods study used a positive deviance approach to understand successful strategies to enhance HPV UTD rates in rural primary care clinics. Clinics with higher HPV UTD rates shared a number of organizational and behavioral features despite facing similar barriers experienced by other rural clinics. Clinics with lower HPV UTD rates revealed an absence of systematized approaches that were present in higher performing clinics. The strategies identified in higher performing clinics are not innovative in themselves.28,48–50 However, the presence of these organizational and behavioral features in higher performing clinics highlights these strategies as powerful forces that may be necessary or perhaps even sufficient to overcome barriers to administering the HPV vaccine in rural primary care clinics.
Higher performing clinics had an established foundation for delivering the HPV vaccine, including securing staff time for conducting immunization work. These clinics devoted substantial staffing resources to attain higher HPV UTD rates. Adding staff time into the mix of a primary care clinic may not be financially or logistically feasible for many rural clinics, but creating efficiencies in workflows can open up time for clinical staff to conduct administrative immunization activities. Efficiencies are created when quality improvement initiatives are established, including the CDC’s immunization quality improvement program.51–53
Higher performing clinics strongly communicated the importance of the vaccine, though intermittent outdated messaging related to HPV and the purpose of the vaccine was observed. Communication or education strategies should focus on the importance of cancer prevention for both male and female patients, as called for in recent studies.30,54 Promising research utilizes social media to create “virtual focus groups” to craft HPV messaging (including clinician communication) for rural residents, which deserves further investigation.55
Limitations
This study has a few notable limitations. As with any qualitative observational data collection, these data represent a snapshot from a single point in time and were limited to the number of adolescent patient visits that occurred during observations. The research team attempted to mitigate this in a number of ways, including conducting pre-visit interviews designed to identify the optimal days and times to observe adolescent visits, which staff to shadow, and best physical location to maximize the observations. Additionally, there is always the risk of personal biases in qualitative research. This was mitigated in two major ways: Qualitative team members were trained in reflexivity and observational techniques prior to data collection, and qualitative team members consistently member checked during observation and routinely consulted with the multidisciplinary team members during observation and analysis. The composition of the multidisciplinary team and multiple methods of data collection was an additional way of triangulating the data sources, investigators, and methods.45,56
Although a number of promising approaches were identified, comparative effectiveness was not assessed. Some of these methods may have been more impactful among various populations, though it was beyond the scope of this study to explore these associations. Despite this, the consistency with which many factors were used by higher performing clinics suggests that the evidence strongly supports multi-intervention approach strategies.
This study is focused solely on clinic-level barriers, but barriers are encountered at different levels. To attend to the disparities faced by rural patients, future research should include multilevel approaches to improve rural HPV vaccine delivery. Multilevel, culturally appropriate interventions have been called for by researchers and policymakers.57,58
CONCLUSIONS
A positive deviance approach was successful in identifying characteristics associated with higher HPV UTD rates in rural primary care clinics and demonstrated that these characteristics are largely similar to those known to facilitate higher vaccination rates overall. In addition to providing new information about what actually happens in rural and frontier clinics, this study highlights these strategies as potentially powerful targets for future intervention work to close rural–urban disparities in HPV vaccination.
Supplementary Material
ACKNOWLEDGMENTS
The authors appreciate the time and insight of the clinic members and who participated in this research, as well as the patients and their families who were willing to be observed. Dr. Paul Darden and the Oregon Immunization Program staff were helpful in providing feedback and suggestions with interpreting the findings. In addition, the authors would like to acknowledge the work of Rex Larsen and Steele Valenzuela, who helped interpret ALERT Immunization Information System data. This study was supported by the American Cancer Society (RSG-18–022-01-CPPB). Dr. Davis’ time was supported in part by an NCI K07 award (1K07CA211971–01A1). The content provided is solely the responsibility of the authors and does not necessarily represent the official views of the funders. The IRB at Oregon Health & Science University approved this study on September 11, 2018 (#18660). LJF, PAC, BH, and MMD designed the protocol and oversaw data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Preliminary results of this study were presented at the 2019 Society for Applied Anthropology Annual Meeting and the 2019 North American Primary Care Research Group Annual Meeting.
No financial disclosures were reported by the authors of this paper.
Clinical Trials registration title: Increasing Human Papillomavirus (HPV) Immunization Rates: The Rural Adolescent Vaccine Enterprise (RAVE) #NCT03604393, registered July 27, 2018.
REFERENCES
- 1.National Cancer Institute, Center for Cancer Research. The HPV Vaccine. https://ccr.cancer.gov/news/landmarks/article/hpv-vaccine. Published 2019. Accessed July 31, 2019.
- 2.Drolet M, Benard E, Perez N, Brisson M. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet. 2019;394(10197):497–509. 10.1016/S0140-6736(19)30298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. HPV Vaccine Safety and Effectiveness. www.cdc.gov/hpv/parents/vaccinesafety.html. Published 2019. Accessed July 31, 2019.
- 4.Vorsters A, Arbyn M, Baay M, et al. Overcoming barriers in HPV vaccination and screening programs. Papillomavirus Res 2017;4:45–53. 10.1016/j.pvr.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC. About HPV www.cdc.gov/hpv/parents/about-hpv.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fhpv%2Fparents%2Fwhatishpv.html. Published 2019. Accessed July 31, 2019.
- 6.Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2017. MMWR Morb Mortal Wkly Rep 2018;67(33):909–917. 10.15585/mmwr.mm6733a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swiecki-Sikora AL, Henry KA, Kepka D. HPV vaccination coverage among US teens across the rural-urban continuum. J Rural Health. 2019;35(4):506–517. 10.1111/jrh.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaiser Family Foundation. The HPV Vaccine: Access and Use in the U.S www.kff.org/womens-health-policy/fact-sheet/the-hpv-vaccine-access-and-use-in-the-u-s/. Published October 9, 2018. Accessed April 3, 2020.
- 9.Oregon Immunization Program. ALERT Immunization Information System Data. Oregon Health Authority. www.oregon.gov/oha/ph/preventionwellness/vaccinesimmunization/alert/pages/index.aspx. Accessed April 3, 2020.
- 10.CDC, Immunization Information Systems Support Branch, National Center for Immunization and Respiratory Diseases. Clinical Decision Support for Immunization (CDSi): Logic Specification for ACIP Recommendations. www.cdc.gov/vaccines/programs/iis/cdsi.html. Published 2019. Accessed April 3, 2020.
- 11.Mills LA, Head KJ, Vanderpool RC. HPV vaccination among young adult women: a perspective from Appalachian Kentucky. Prev Chronic Dis 2013;10:E17 10.5888/pcd10.120183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanderpool RC, Breheny PJ, Tiller PA, et al. Implementation and evaluation of a school-based human papillomavirus vaccination program in rural Kentucky. Am J Prev Med 2015;49(2):317–323. 10.1016/j.amepre.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Lai D, Ding Q, Bodson J, Warner EL, Kepka D. Factors associated with increased HPV vaccine use in rural-frontier U.S. states. Public Health Nurs 2016;33(4):283–294. 10.1111/phn.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kepka D, Spigarelli MG, Warner EL, Yoneoka Y, McConnell N, Balch A. Statewide analysis of missed opportunities for human papillomavirus vaccination using vaccine registry data. Papillomavirus Res 2016;2:128–132. 10.1016/j.pvr.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham-Erves J, Koyama T, Huang Y, et al. Providers’ perceptions of parental human papillomavirus vaccine hesitancy: cross-sectional study. JMIR Cancer. 2019;5(2):e13832 10.2196/13832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cartmell KB, Young-Pierce J, McGue S, et al. Barriers, facilitators, and potential strategies for increasing HPV vaccination: a statewide assessment to inform action. Papillomavirus Res 2018;5:21–31. 10.1016/j.pvr.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr 2014;168(1):76–82. 10.1001/jamapediatrics.2013.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sussman AL, Helitzer D, Bennett A, Solares A, Lanoue M, Getrich CM. Catching up with the HPV vaccine: challenges and opportunities in primary care. Ann Fam Med 2015;13(4):354–360. 10.1370/afm.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyd ED, Phillips JM, Schoenberger YM, Simpson T. Barriers and facilitators to HPV vaccination among rural Alabama adolescents and their caregivers. Vaccine. 2018;36(28):4126–4133. 10.1016/j.vaccine.2018.04.085. [DOI] [PubMed] [Google Scholar]
- 20.Shipman SA, Lan J, Chang CH, Goodman DC. Geographic maldistribution of primary care for children. Pediatrics. 2011;127(1):19–27. 10.1542/peds.2010-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walling EB, Benzoni N, Dornfeld J, et al. Interventions to improve HPV vaccine uptake: a systematic review. Pediatrics. 2016;138(1):e20153863 10.1542/peds.2015-3863. [DOI] [PubMed] [Google Scholar]
- 22.Wishik SM, Vynckt S. The use of nutritional ‗positive deviants’ to identify approaches for modification of dietary practices. Am J Public Health. 1976;66(1):38–42. 10.2105/ajph.66.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baxter R, Taylor N, Kellar I, Lawton R. What methods are used to apply positive deviance within healthcare organisations? A systematic review. BMJ Qual Saf 2016;25(3):190–201. 10.1136/bmjqs-2015-004386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howell EA, Ahmed ZN, Sofaer S, Zeitlin J. Positive deviance to address health equity in quality and safety in obstetrics. Clin Obstet Gynecol 2019;62(3):560–571. 10.1097/grf.0000000000000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goff SL, Mazor KM, Priya A, Moran M, Pekow PS, Lindenauer PK. Organizational characteristics associated with high performance on quality measures in pediatric primary care: a positive deviance study. Health Care Manage Rev In press. Online May 20, 2019. 10.1097/hmr.0000000000000247. [DOI] [PMC free article] [PubMed]
- 26.Bradley EH, Curry LA, Ramanadhan S, Rowe L, Nembhard IM, Krumholz HM. Research in action: using positive deviance to improve quality of health care. Implement Sci 2009;4:25 10.1186/1748-5908-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsh DR, Schroeder DG, Dearden KA, Sternin J, Sternin M. The power of positive deviance. BMJ 2004;329(7475):1177–1179. 10.1136/bmj.329.7475.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perkins RB, Fisher-Borne M, Brewer NT. Engaging parents around vaccine confidence: proceedings from the National HPV Vaccination Roundtable meetings. Hum Vaccin Immunother. 2019;15(7‒8):1639–1640. 10.1080/21645515.2018.1520592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niccolai LM, Hansen CE. Practice- and community-based interventions to increase human papillomavirus vaccine coverage: a systematic review. JAMA Pediatr 2015;169(7):686–692. 10.1001/jamapediatrics.2015.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wheldon CW, Krakow M, Thompson EL, Moser RP. National trends in human papillomavirus awareness and knowledge of human papillomavirus–related cancers. Am J Prev Med 2019;56(4):e117–e123. 10.1016/j.amepre.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Nagykaldi Z, Mold JW, Robinson A, Niebauer L, Ford A. Practice facilitators and practice-based research networks. J Am Board Fam Med 2006;19(5):506–510. 10.3122/jabfm.19.5.506. [DOI] [PubMed] [Google Scholar]
- 32.Creswell JW. Steps in conducting a scholarly mixed methods study. University of Nebraska - Lincoln; 2013. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1047&context=dberspeakers. Accessed April 3, 2020. [Google Scholar]
- 33.NIH Office of Behavioral Science. Best practices for mixed methods research in the health sciences, 2nd ed. Bethesda, MD: NIH; 2018. www.obssr.od.nih.gov/wp-content/uploads/2018/01/Best-Practices-for-Mixed-Methods-Research-in-the-Health-Sciences-2018-01-25.pdf. Accessed April 3, 2020. [Google Scholar]
- 34.Ivankova NV, Creswell JW, Stick SL. Using mixed-methods sequential explanatory design: from theory to practice. Field Methods. 2006;18(1):3–20. 10.1177/1525822x05282260. [DOI] [Google Scholar]
- 35.Leech NL, Onwuegbuzie AJ. A typology of mixed methods research designs. Qual Quant 2009;43(2):265–275. 10.1007/s11135-007-9105-3. [DOI] [Google Scholar]
- 36.Carney PA, Hatch B, Stock I, et al. A stepped-wedge cluster randomized trial designed to improve completion of HPV vaccine series and reduce missed opportunities to vaccinate in rural primary care practices. Implement Sci 2019;14:30 10.1186/s13012-019-0871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robison SG. Addressing immunization registry population inflation in adolescent immunization rates. Public Health Rep 2015;130(2):161–166. 10.1177/003335491513000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.U.S. Department of Agriculture. Rural-Urban Commuting Area Codes. www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/. Published 2019. Accessed August 1, 2019.
- 39.Oregon Office of Rural Health. About Rural and Frontier Data: Oregon Zip Codes, Towns, Cities, and Service Areas and their ORH Urban/Rural/Frontier Designation. www.ohsu.edu/oregon-office-of-rural-health/about-rural-frontier/data. Published 2018. Accessed August 1, 2019.
- 40.Robison SG. Addressing immunization registry population inflation in adolescent immunization rates. Public Health Rep 2015;130(2):161–166. 10.1177/003335491513000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3(2):77–101. 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- 42.CDC. (IQIP) Immunization Quality Improvement for Providers. www.cdc.gov/vaccines/programs/iqip/index.html. Published 2019. Accessed September 23, 2019.
- 43.Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant 2018;52(4):1893–1907. 10.1007/s11135-017-0574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leung L Validity, reliability, and generalizability in qualitative research. J Family Med Prim Care. 2015;4(3):324–327. 10.4103/2249-4863.161306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Creswell JW, Miller DL. Determining validity in qualitative inquiry. Theory Pract 2000;39(3):124–130. 10.1207/s15430421tip3903_2. [DOI] [Google Scholar]
- 46.Morse JM. Critical analysis of strategies for determining rigor in qualitative inquiry. Qual Health Res 2015;25(9):1212–1222. 10.1177/1049732315588501. [DOI] [PubMed] [Google Scholar]
- 47.Spillett MA. Peer debriefing: who, what, when, why, how. Academic Exchange Quarterly. 2003.
- 48.Chuang E, Cabrera C, Mak S, Glenn B, Hochman M, Bastani R. Primary care team- and clinic level factors affecting HPV vaccine uptake. Vaccine. 2017;35(35 Pt B):4540–4547. 10.1016/j.vaccine.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 49.Escoffery C, Riehman K, Watson L, et al. Facilitators and barriers to the implementation of the HPV VACs (Vaccinate Adolescents Against Cancers) program: a consolidated framework for implementation research analysis. Prev Chronic Dis 2019;16:E85 10.5888/pcd16.180406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cates JR, Diehl SJ, Fuemmeler BF, et al. Toward optimal communication about HPV vaccination for preteens and their parents: evaluation of an online training for pediatric and family medicine health care providers. J Public Health Manag Pract 2020;26(2):159–167. 10.1097/phh.0000000000001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Askelson NM, Ryan G, Seegmiller L, Pieper F, Kintigh B, Callaghan D. Implementation challenges and opportunities related to HPV vaccination quality improvement in primary care clinics in a rural state. J Community Health. 2019;44(4):790–795. 10.1007/s10900-019-00676-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gilkey MB, Dayton AM, Moss JL, et al. Increasing provision of adolescent vaccines in primary care: a randomized controlled trial. Pediatrics 2014;134(2):e346–e353. 10.1542/peds.2013-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gilkey MB, Moss JL, Roberts AJ, Dayton AM, Grimshaw AH, Brewer NT. Comparing in-person and webinar delivery of an immunization quality improvement program: a process evaluation of the adolescent AFIX trial. Implement Sci 2014;9:21 10.1186/1748-5908-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daley EM, Vamos CA, Thompson EL, et al. The feminization of HPV: how science, politics, economics and gender norms shaped U.S. HPV vaccine implementation. Papillomavirus Res 2017;3:142–148. 10.1016/j.pvr.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Virginia Commonwealth University. Virtual focus groups’ uncover clues to rural and urban HPV vaccination disparities. Science Daily. www.sciencedaily.com/releases/2019/03/190313103207.htm. Published March 13, 2019. Accessed September 9, 2019.
- 56.Carter N, Bryant-Lukosius D, DiCenso A, Blythe J, Neville AJ. The use of triangulation in qualitative research. Oncol Nurs Forum. 2014;41(5):545–547. 10.1188/14.onf.545-547. [DOI] [PubMed] [Google Scholar]
- 57.Vanderpool RC, Stradtman LR, Brandt HM. Policy opportunities to increase HPV vaccination in rural communities. Hum Vaccin Immunother. 2019;15(7‒8):1527–1532. 10.1080/21645515.2018.1553475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vamos CA, Vazquez-Otero C, Kline N, et al. Multi-level determinants to HPV vaccination among Hispanic farmworker families in Florida. Ethn Health. In press. Online September 27, 2018 10.1080/13557858.2018.1514454. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


