Abstract
Background:
Gene-obesogenic environment interactions influence body mass index (BMI) across the life-course; however, limited research examines how these interactions may differ by race and sex.
Methods:
Utilizing mixed-effects models, we examined the interaction effects of a polygenic risk score (PGS) generated from BMI-associated single nucleotide polymorphisms, and environmental factors, including age, physical activity, alcohol intake and childhood socioeconomic status on measured longitudinal BMI from the Health and Retirement Study (HRS). HRS is a population representative survey of older adults in the United States. This study used a sub-sample of genotyped Black (N=1,796) and White (N=4,925) men and women (50–70 years) with measured BMI.
Results:
Higher PGS was associated with higher BMI. The association between PGS and BMI weakened as individuals aged among White men (Pinteraction=0.0383) and White women (Pinteraction=0.0514). The mean BMI difference between the 90th and 10th PGS percentile was 4.25 kg/m2 among 50-year old White men, and 3.11 kg/m2 among the 70-year old’s, i.e. a 1.14 kg/m2 (95%CI: −0.27, 2.82) difference. The difference among 50- and 70-year old White women was 1.34 kg/m2 (95%CI: 0.09, 2.60). Additionally, the protection effect of physical activity was stronger among White women with higher PGS (Pinteraction=0.0546). Vigorous physical activity (compared to never) was associated with 1.66 kg/m2 (95%CI: 1.06, 2.29) lower mean BMI among those in the 90th PGS percentile, compared to 0.83 kg/m2 (95%CI: 0.37, 1.29) lower among those in the 10th PGS percentile. Interactions were also observed between both PGS and alcohol intake among White men (Pinteraction=0.0034) and women (Pinteraction=0.0664) and Black women (Pinteraction=0.0108), and PGS and childhood socioeconomic status among White women (Pinteraction=0.0007).
Conclusion:
Our findings reinforce the importance of physical activity among those with an elevated genetic risk; additionally, other detected interactions may underscore the influence of broader social environments on obesity-promoting genes.
Introduction
Elevated body mass index (BMI) is a strongly influential component to the emergence of many adverse health conditions, including type 2 diabetes1, cardiovascular diseases2, and certain cancers3, presenting a significant burden on the healthcare system.4, 5 Obese individuals, typically measured by BMI, have an increased risk of all-cause mortality relative to those in the normal weight range, especially among those within the class 2 (BMI = 35.0–39.9 kg/m2) and class 3 (BMI ≥ 40.0 kg/m2) subclassifications.6 In the US, 35–40% of all adults’ BMI fall within the obesity range, with 5.5–9.9% belonging to class 3, representing the highest risk group for morbidity and mortality.7 Moreover, while some researchers report a potential protective effect of overweight status (BMI = 25.0–29.9 kg/m2),6 a recent large meta-analysis observed an increased risk of all-cause mortality, cardiovascular disease, and cancer in BMI categories as low as 21–25 kg/m2.4
Since the discovery of the first BMI-related gene, FTO (fat-mass and obesity-related gene), through the introduction of genome-wide association studies (GWAS), variations in BMI have been linked to tens of thousands of single nucleotide polymorphisms (SNPs).8 A recent meta-analysis of GWAS identified 97 common BMI-increasing SNPs from samples of individuals of European ancestry8, 9 However, despite the influx of these early discoveries from GWAS, individual SNPs only account for a small proportion of monogenic forms of disease, or about 3–4% of the total variation of BMI. It is now generally recognized that the genetics of BMI reflect the combined genetic variations of multiple risk alleles, leading to the use of polygenic risk scores (PGS) in evaluating the combined effects of known BMI-elevating genetic variants.10
Much of the global obesity epidemic may be attributable to both the interaction and independent effects of environmental/lifestyle and heritable/genetic factors; only in the past few years have investigators examined the interaction between GWAS-derived genetics and environmental/lifestyle factors, such as physical activity,11, 12 diet,13–15 sleep charactistics,16 and other obesogenic factors.17–19 For instance, Rask-Andersen et al.20 reported that both higher frequencies of alcohol intake and physical activity may mitigate the effects of genetic predisposition to higher BMI. Additionally, Tyrrell et al.21 observed that a higher degree of social deprivation and physical inactivity may exacerbate genetic susceptibility to higher BMI. Recent findings from the Nurses’ Health Study and the Health Professionals Follow-Up Study report sex differences in the relationship between genetic risk and BMI, possibly elucidating an underlying mechanism for the widely-recognized adiposity differences between men and women.22 Moreover, a prior gene-environment interaction study reported that, among women, alcohol intake associated with a higher waist circumference-adjusted BMI; however, higher polygenic risk was associated with a significantly lower body weight for these women.23 While epidemiologic studies report that many racial/ethnic minority groups, including Black and Latino Americans, are disproportionately affected by the recent upwards obesity trends compared to Whites, these groups remain significantly underrepresented in both the GWAS and gene-environment studies of BMI, with most large cohorts consisting primarily of European-ancestry samples.24
The U.S. Health and Retirement Study (HRS) offers an excellent opportunity to examine gene-environment interaction across race/ethnicity groups, since it includes nationally representative samples of Black and White Americans. While researchers have examined interactions between PGS and other obesogenic factors among Black and White HRS participants, their scope has been limited to factors such as cohort25 and psychosocial26 interactions. They have yet to examine the contribution of other modifiable BMI-elevating lifestyle factors. Specifically, the main and PGS interaction effects of alcohol use, smoking, and physical activity have yet to be examined within the HRS cohort, with regards to BMI. Moreover, to our knowledge, no literature exists on the moderating effects of both sex and ethnicity within the HRS, both of which have been shown to modify the obesogenic effects of genetic and environmental factors.24, 27 This study thus aims to examine the independent and interaction effects of modifiable lifestyle factors and genetic risk on BMI within ethnicity- and sex-stratified samples of older Black and White men and women from the HRS. In the current study, we hypothesize that the PGS influences mean BMI differently by ethnicity and sex, and that the interplay of environmental and polygenic factors contributes differentially to BMI among older adults.
Methods
The Health and Retirement Study
The Health and Retirement Study is a nationally representative longitudinal cohort study aimed at examining the health outcomes of approximately 43,000 United States’ men and women older than 50 years of age at recruitment, and their spouses. Recruitment, sampling, and overall population characteristics have been previously summarized elsewhere.28, 29 Sampling for the HRS is built upon a complex multi-stage area probability design utilizing geographical stratification and clustering with oversampling of Black Americans. The HRS sample consists of seven continuing cohorts including the initial HRS cohort, (born 1931–41), Asset and Health Dynamics Among the Oldest Old (AHEAD, born 1890–1923), the Children of the Depression (CODA, born 1924–30), and the War Babies (WB, born 1942–47), Early Baby Boomers (EBB, born 1948–53), Mid Baby Boomers (MBB, born 1954–59), and Late Baby Boomers (LBB, born 1960–1965). HRS conducted face-to-face or phone core interviews, during which participants were asked questions about finances, health status and behaviors, marital/family status, and social support systems. A random half sample is then followed-up biennially for core interviews. Starting in 2006, HRS initiated Enhanced Face-to-Face Interview (EFTF) for a random half of the core interview samples. The EFTF interview includes a set of anthropometric measurements, physical performance tests, and blood and saliva samples for genotyping, etc. The other half sample was selected for the next follow-up EFTF interview and so forth. The anthropometric measurements of EFTF sample were repeatedly measured an average of 2.2 times per participant.
The HRS is sponsored by the National Institute of Aging (U01AG009740) and is conducted by the University of Michigan. The current study utilized publicly accessible, de-identified data from HRS, approved by the institutional review board at the University of Hawaiʻi (approval number CHS23551).
Analytic Sample
The derivation of the final analytic sample is presented in Figure 1. We began with a sample of 15,190 HRS participants (12,090 White and 3,100 Black) with genetic data collected between 2006 and 2012. The final analytic sample consists of 4,925 White (2,115 men and 2,810 women) and 1,796 Black (698 men and 1,098 women) after excluding 586 participants with missing BMI measurements, 7,246 older cohorts (AHEAD, HRS and CODA), 535 who were 20 to 49 years old, and 103 who had missing covariates. We limited our study to the three younger cohorts (WB, EBB and MBB) to mitigate confounding by cohort effects, and survival bias.30
Figure 1.
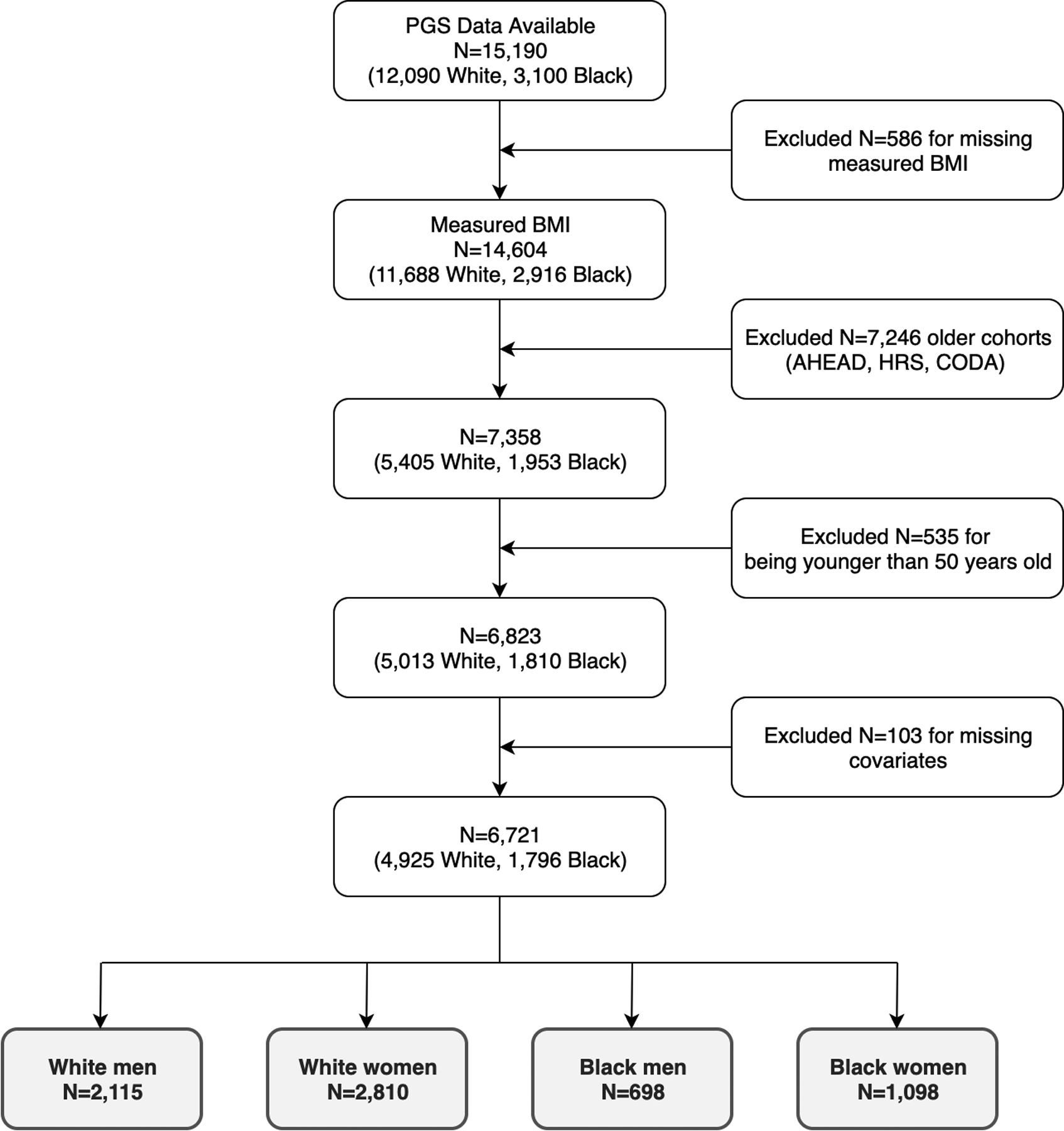
Analytic Sample Derivation from the Health and Retirement Study
Measures
Our main outcome was calculated BMI from repeated measurements of height and weight; the HRS objectively measured weights (in kilograms) and heights (in meters) during home visits between 2006–2016 to calculate the BMI (kg/m2). To assess height, participants were asked to stand against a wall without shoes while the interviewer marked the wall and then measured the distance from the floor to the marking. Weight was ascertained by asking participants to remove their shoes and heavy items/clothing and step on a Healthometer 830KL scale. Salivary DNA samples were collected for genotyping during the same visit using the mouthwash collection method (buccal cell swab) in 2006; in 2008, and thereafter, an Oragene self-collection kit was used to collect salivary DNA.
Socio-demographic characteristics, including age, sex, education level, adult poverty ratio, childhood socioeconomic status (cSES), and the health measure of depression (assessed through Center for Epidemiologic Studies Depression [CESD-8] scale), were self-reported directly from the HRS core interview. Self-reported sex included dichotomous levels of ‘male’ or ‘female’. Education level included ‘less than high school’, ‘general education development certification (GED)’, ‘high school diploma’, ‘some college-level education’, and ‘greater than some college-level education’. Adult poverty ratio was calculated from household income, accounting for self-reported and spousal incomes, from wave- and census-track specific poverty threshold levels used by the U.S. Census Bureau; whereas, cSES was derived directly from the core survey item asking ‘now think about your family when you were going up, from birth to age 16. Would you say your family during that time was pretty well off financially, about average, or poor?’. Finally, HRS used a modified eight-item CESD scale examining depressive symptoms over the past week, which has acceptable internal consistency (α = 0.81–0.83).31 Adult poverty ratio, cSES, and depression were considered as potential covariates because previous studies have shown their association with BMI32–34 and suggested that boarder social environments may influence the effects of obesity-promoting genes.35 Moreover, prior studies report significant interactions between adult deprivation, a similar covariate to adult poverty ratio, and polygenic risk on BMI.21
The HRS core interview provided data on obesogenic lifestyle factors, including physical activity level, alcohol consumption, and smoking behaviors. Physical activity was ascertained from self-reported frequency of participants’ engagement in housework, aerobics, running, swimming, bicycling, or other physical labor, and recoded into ‘never/some’, ‘two or more light’, ‘two or more moderate’, and ‘two or more vigorous’ activities per week. Alcohol use was determined from self-reported alcoholic drinks per day (zero, one, two, and three or more). Lastly, smoking was ascertained from self-reported tobacco use (never, past, and current). Physical activity, alcohol intake, and tobacco smoking were considered as covariates because of prior studies reporting significant interactions with polygenic risk on BMI.11, 27, 36
PGS derivation
PGSs for BMI were computed by the HRS research team using results from a 2015 study conducted by the Genetic Investigation of ANthropometric Traits (GIANT) consortium.37 Weighted sum scores were calculated using HRS available SNPs in the PGS that overlap between the GIANT and the HRS genetic data.38 Weights were defined by the β-coefficient estimate from the GIANT GWAS meta-analysis conducted on 332,154 individuals of European ancestry. If the β-coefficient value was negative, the β measures were converted to positive values and the reference allele flipped to represent phenotype-increasing PGSs. PGSs and 10 ancestry principle components (PCs) were computed for both the White and Black groups, separately. We scaled the PGS to rank percentiles (range 0 to 1) for Black and White men and women separately.
Statistical analyses
Descriptive statistics were calculated to summarize baseline sample characteristics. We utilized linear mixed-effects models (LMMs) with random effects to account for the correlations of repeated measurements of BMI nested within participants.39 We performed bivariate LMM analyses for PGS rank percentile, demographics, and obesogenic lifestyle factors, respectively; and multivariate LMM models for PGS rank percentiles adjusting for all other variables. Interactions between the PGS rank percentiles and the demographic/obesogenic environment variables on BMI were tested by including the respective interaction terms in the multivariate LMM models. In the interaction models, we also tested non-linear effects of age on BMI using cubic-polynomial functions of age (i.e., age, age2 and age3) (Supplementary A.1). To build the most parsimonious interaction models, we applied both stepwise and backward elimination model building strategies manually for model selections. Generalized linear hypothesis (GLH) testing method was used for model selections and contrasts of the interaction effects (Supplementary A.2).40 Bootstrap method with 2000 replications was applied for complex contrasts in the interactions, for example, to assess if the association between BMI and PGS was the same at age 50 and age 70 (Supplementary A.3).41
For all models with PGS, 10 ancestry PCs were included to account for population stratification and ancestry differences in genetic structures. Separate analyses were performed for White and Black men and women. All analyses were conducted using statistical software R version 3.5.2. and p-values are two-sided.
Results
Baseline Characteristics
Table 1 presents the baseline sample characteristics stratified by race (Black and White) and sex (men and women). The mean BMI was similar between men (29.8 kg/m2) and women (29.9 kg/m2); however, the proportion of normal-BMI (<25.0kg/m2) White women (26.8%) was greater than normal-BMI White men (16.0%) at baseline. Among Black participants, the mean BMI measurement differed between men and women at baseline, with an average of 33.2 kg/m2 among women, compared to 29.1 kg/m2 among men.
Table 1.
Baseline Sample Characteristics for White and Black men and women study participants.
| Variables | White | Black | |||||
|---|---|---|---|---|---|---|---|
| Men (N=2,115) | Women (N=2,810) | p-value | Men (N=698) | Women (N=1,098) | p-value | ||
| Range | mean (SD) | mean (SD) | mean (SD) | mean (SD) | |||
| Baseline age (years) | 53–73 | 57.5 (4.3) | 57.6 (4.6) | 0.4534 | 57.2 (4.2) | 57.1 (4.1) | 0.5883 |
| BMI (kg/m2) | 15–70 | 29.8 (5.1) | 29.9 (7.0) | 0.4902 | 29.1 (5.7) | 33.2 (7.4) | <.0001 |
| PGS rank percentile | 0–1 | 0.5 (0.3) | 0.5 (0.3) | 0.9399 | 0.5 (0.3) | 0.5 (0.3) | 0.6213 |
| CESD | 0–8 | 1.1 (1.8) | 1.4 (2.0) | <.0001 | 1.8 (2.0) | 2.1 (2.3) | 0.0077 |
| Levels | N (%) | N (%) | N (%) | N (%) | |||
| BMI Categories | Normal | 338 (16.0%) | 753 (26.8%) | <.0001 | 166 (23.8%) | 137 (12.5%) | <.0001 |
| Overweight | 879 (41.6%) | 848 (30.2%) | 246 (35.2%) | 250 (22.8%) | |||
| Obese | 898 (42.5%) | 1,209 (43.0%) | 286 (41.0%) | 711 (64.8%) | |||
| Cohort | War babies | 627 (29.6%) | 955 (34.0%) | 0.0024 | 112 (16.0%) | 194 (17.7%) | 0.55 |
| Early Baby boomers | 776 (36.7%) | 926 (33.0%) | 267 (38.3%) | 427 (38.9%) | |||
| Mid Baby Boomers | 712 (33.7%) | 929 (33.1%) | 319 (45.7%) | 477 (43.4%) | |||
| Education | < High school | 128 (6.1%) | 194 (6.9%) | <.0001 | 141 (20.2%) | 214 (19.5%) | 0.3915 |
| High school/GED | 620 (29.3%) | 986 (35.1%) | 252 (36.1%) | 360 (32.8%) | |||
| College 1–3 years | 619 (29.3%) | 826 (29.4%) | 203 (29.1%) | 351 (32.0%) | |||
| College 4+ years | 748 (35.4%) | 804 (28.6%) | 102 (14.6%) | 173 (15.8%) | |||
| Poverty Ratio | Under poverty | 95 (4.5%) | 139 (4.9%) | 0.0012 | 131 (18.8%) | 261 (23.8%) | 0.0107 |
| 1 to 2.9 | 393 (18.6%) | 637 (22.7%) | 246 (35.2%) | 401 (36.5%) | |||
| 3 or higher | 1,627 (76.9%) | 2,034 (72.4%) | 321 (46.0%) | 436 (39.7%) | |||
| Childhood SES | Poor | 417 (19.7%) | 594 (21.1%) | 0.4506 | 277 (39.7%) | 397 (36.2%) | 0.1994 |
| Average | 1,501 (71.0%) | 1,966 (70.0%) | 373 (53.4%) | 634 (57.7%) | |||
| Well off | 197 (9.3%) | 250 (8.9%) | 48 (6.9%) | 67 (6.1%) | |||
| Smoking Status | Non-smoker | 810 (38.3%) | 1,343 (47.8%) | <.0001 | 209 (29.9%) | 481 (43.8%) | <.0001 |
| Current smoker | 444 (21.0%) | 526 (18.7%) | 251 (36.0%) | 285 (26.0%) | |||
| Past smoker | 861 (40.7%) | 941 (33.5%) | 238 (34.1%) | 332 (30.2%) | |||
| Alcohol intake | Never | 582 (27.5%) | 1,057 (37.6%) | <.0001 | 257 (36.8%) | 550 (50.1%) | <.0001 |
| 1/day | 1,025 (48.5%) | 1,508 (53.7%) | 322 (46.1%) | 484 (44.1%) | |||
| 2/day | 278 (13.1%) | 177 (6.3%) | 71 (10.2%) | 48 (4.4%) | |||
| 3 or more/day | 230 (10.9%) | 68 (2.4%) | 48 (6.9%) | 16 (1.5%) | |||
| Physical Activity* | Some or Never | 411 (19.4%) | 394 (14.0%) | <.0001 | 221 (31.7%) | 305 (27.8%) | <.0001 |
| Light | 257 (12.2%) | 623 (22.2%) | 73 (10.5%) | 285 (26.0%) | |||
| Moderate | 690 (32.6%) | 1,048 (37.3%) | 188 (26.9%) | 329 (30.0%) | |||
| Vigorous | 757 (35.8%) | 745 (26.5%) | 216 (30.9%) | 179 (16.3%) | |||
Physical Activity: twice or more light, moderate and vigorous physical activity per week; Some: once per week or per month any type of physical activities.
Acronyms: BMI: Body Mass Index; PGS: Polygenic Risk Score; GED: General Educational Development/Certificate of High School Equivalency; CESD: Center for Epidemiologic Studies Depression Scale, 8-item; SES: Socioeconomic Status; SD: Standard Deviation.
Main Effects of the PGS across Ethnicity and Sex
Supplementary Tables S1 and S2 show results from bivariate and multivariate main-effects LMM models for White and Black men and women. The β-coefficients for PGS rank percentiles were similar in the bivariate and multivariate models. In the multivariate model, PGS was associated with 4.78 kg/m2 greater BMI (95%CI: 4.04, 5.52, p<0.0001) among White men, and 7.52 kg/m2 greater BMI (95%CI: 6.68, 8.37, p<0.0001) among White women. Among Black men and women, the changes in mean BMI by PGS were 4.14 (95%CI: 2.34, 5.94, p<0.0001) and 2.95 (95%CI: 1.12, 4.78, p=0.0016), respectively.
PGS Interaction Models
Interactions were detected between PGS and age, physical activity, alcohol intake and cSES. Table 2 presents all interaction effects for demographic and behavioral covariates across ethnic- and sex-specific subsamples.
Table 2.
Regression coefficients for BMI estimated from the final interaction LMM models that include interaction between PGS percentile (0–1) and age, physical activities, alcohol intake and childhood SES for White and Black men and women.
| White Men | White Women | Black Men | Black Women | |||||
|---|---|---|---|---|---|---|---|---|
| (95% CI) | p-value | (95% CI) | p-value | (95% CI) | p-value | (95% CI) | p-value | |
| Intercept | 28.19 (26.74, 29.63) | <0.0001 | 28.51 (27.01, 30.00) | <0.0001 | 27.73 (21.56, 33.89) | <0.0001 | 33.40 (27.68, 39.11) | <0.0001 |
| PGS and Age | ||||||||
| PGS | 3.76 (2.00, 5.52) | <0.0001 | 5.99 (4.08, 7.90) | <0.0001 | 3.15 (−6.59, 12.89) | 0.5262 | 7.85 (−1.31, 17.00) | 0.0929 |
| Age | 28.43 (14.17, 42.69) | <0.0001 | 25.48 (8.04, 42.93) | 0.0042 | 0.01 (−0.08, 0.10) | 0.8163 | 0.01 (−0.08, 0.09) | 0.9043 |
| Age2 | 3.11 (−7.60, 13.83) | 0.5689 | −12.66 (−24.95, −0.37) | 0.0435 | ||||
| Age3 | 3.88 (−5.78, 13.54) | 0.4312 | 3.86 (−7.34, 15.05) | 0.4995 | ||||
| PGS*Age | −25.77 (−50.14, −1.39) | 0.0383 | −29.59 (−59.35, 0.18) | 0.0514 | 0.00 (−0.15, 0.16) | 0.9496 | −0.09 (−0.24, 0.05) | 0.1883 |
| PGS*Age2 | −9.53 (−27.91, 8.85) | 0.3094 | −1.60 (−23.16, 19.96) | 0.8844 | ||||
| PGS*Age3 | −4.04 (−20.56, 12.49) | 0.6321 | −9.30 (−28.81, 10.20) | 0.3498 | ||||
| PGS and Physical Activity | ||||||||
| Some or Never (Ref) | ||||||||
| Light | −0.13 (−0.71, 0.46) | 0.6651 | −0.52 (−1.06, 0.03) | 0.0622 | −0.27 (−1.45, 0.91) | 0.6543 | −0.40 (−1.29, 0.50) | 0.384 |
| Moderate | −0.05 (−0.56, 0.46) | 0.8344 | −0.46 (−0.99, 0.06) | 0.0846 | −0.06 (−0.96, 0.85) | 0.9015 | −0.16 (−1.03, 0.72) | 0.7228 |
| Vigorous | −0.43 (−0.96, 0.11) | 0.1213 | −0.72 (−1.33, −0.12) | 0.0191 | −0.89 (−1.88, 0.11) | 0.0813 | −0.92 (−1.99, 0.15) | 0.0925 |
| PGS*Light | 0.22 (−0.80, 1.25) | 0.6691 | 0.49 (−0.44, 1.41) | 0.3034 | 0.32 (−1.79, 2.43) | 0.7679 | 0.76 (−0.78, 2.30) | 0.336 |
| PGS*Moderate | −0.04 (−0.91, 0.82) | 0.9222 | −0.67 (−1.59, 0.26) | 0.1573 | −0.49 (−2.07, 1.10) | 0.5458 | −0.12 (−1.63, 1.39) | 0.8799 |
| PGS*Vigorous | −0.21 (−1.13, 0.70) | 0.6471 | −1.04 (−2.10, 0.02) | 0.0546 | 0.71 (−1.02, 2.44) | 0.4194 | 0.15 (−1.68, 1.97) | 0.8758 |
| PGS and Alcohol Intake | ||||||||
| Never (Ref) | ||||||||
| 1/day | −0.68 (−1.26, −0.11) | 0.0199 | 0.24 (−0.30, 0.77) | 0.3873 | 0.02 (−1.09, 1.14) | 0.9663 | 0.50 (−0.42, 1.43) | 0.2836 |
| 2/day | −0.67 (−1.43, 0.09) | 0.0832 | −0.04 (−1.00, 0.92) | 0.9328 | 0.85 (−0.68, 2.38) | 0.2773 | 0.48 (−1.50, 2.47) | 0.634 |
| 3 or more/day | −0.11 (−0.97, 0.75) | 0.8052 | 0.54 (−0.89, 1.97) | 0.4598 | 1.72 (−0.08, 3.51) | 0.0604 | −1.92 (−4.75, 0.91) | 0.1839 |
| PGS*1/day | 1.44 (0.48, 2.41) | 0.0034 | −0.85 (−1.75, 0.06) | 0.0664 | 0.60 (−1.24, 2.44) | 0.5258 | −2.07 (−3.65, −0.48) | 0.0108 |
| PGS*2/day | 1.08 (−0.23, 2.39) | 0.1068 | −0.74 (−2.36, 0.88) | 0.3684 | −1.06 (−3.74, 1.62) | 0.437 | −2.16 (−5.68, 1.36) | 0.2282 |
| PGS*3+/day | 0.21 (−1.30, 1.72) | 0.7806 | −2.49 (−5.10, 0.12) | 0.0619 | −3.99 (−7.23, −0.75) | 0.0158 | 1.91 (−3.29, 7.11) | 0.4723 |
| PGS and Childhood SES | ||||||||
| Poor (Ref) | ||||||||
| Average | −0.65 (−1.70, 0.40) | 0.2249 | −1.13 (−2.30, 0.03) | 0.0565 | −1.50 (−3.19, 0.18) | 0.0809 | −1.29 (−3.13, 0.55) | 0.1689 |
| Well off | −1.52 (−3.12, 0.08) | 0.0624 | −1.38 (−3.22, 0.46) | 0.1422 | −1.09 (−4.23, 2.04) | 0.4939 | −1.71 (−5.35, 1.93) | 0.3582 |
| PGS*Average | 0.21 (−1.55, 1.97) | 0.8164 | 3.40 (1.43, 5.38) | 0.0007 | 1.42 (−1.46, 4.31) | 0.3339 | 2.37 (−0.73, 5.48) | 0.1344 |
| PGS*Well off | 0.61 (−2.17, 3.40) | 0.6651 | 0.77 (−2.42, 3.96) | 0.6379 | 0.42 (−4.84, 5.68) | 0.8756 | 2.05 (−4.61, 8.72) | 0.5462 |
| Cohort | ||||||||
| War babies (Ref) | ||||||||
| Early Baby Boomers | 0.27 (−0.25, 0.78) | 0.3119 | 0.19 (−0.39, 0.77) | 0.5257 | −0.73 (−1.94, 0.48) | 0.2397 | 0.54 (−0.69, 1.77) | 0.3891 |
| Mid Baby Boomers | 0.60 (0.04, 1.15) | 0.0347 | 0.39 (−0.22, 1.00) | 0.2118 | 0.11 (−1.15, 1.37) | 0.866 | 1.08 (−0.19, 2.36) | 0.0959 |
| Education | ||||||||
| < High school (Ref) | ||||||||
| High school/GED | 0.82 (−0.09, 1.74) | 0.0773 | 0.05 (−0.92, 1.02) | 0.9206 | 0.70 (−0.41, 1.80) | 0.2183 | −0.59 (−1.81, 0.62) | 0.3381 |
| College 1–3 years | 0.87 (−0.05, 1.79) | 0.0644 | −0.58 (−1.58, 0.42) | 0.254 | 1.39 (0.20, 2.59) | 0.0222 | −0.75 (−2.00, 0.49) | 0.2365 |
| College 4+ years | 0.43 (−0.51, 1.36) | 0.3722 | −1.97 (−2.99, −0.94) | 0.0002 | 1.82 (0.39, 3.26) | 0.0127 | −0.78 (−2.27, 0.70) | 0.3011 |
| Poverty Ratio | ||||||||
| Under poverty (Ref) | ||||||||
| 1 to 2.9 | 0.26 (−0.20, 0.72) | 0.2754 | 0.09(−0.34, 0.53) | 0.6717 | 0.27 (−0.26, 0.79) | 0.3151 | −0.15 (−0.59, 0.29) | 0.5003 |
| 3 or higher | 0.15 (−0.32, 0.63) | 0.5258 | −0.03(−0.48, 0.42) | 0.9011 | 0.52 (−0.10, 1.14) | 0.101 | −0.28 (−0.83, 0.26) | 0.3124 |
| CESD | −0.04 (−0.10, 0.02) | 0.211 | 0.03(−0.02, 0.09) | 0.2181 | −0.02 (−0.13, 0.09) | 0.7484 | −0.06 (−0.15, 0.04) | 0.2247 |
| Smoking status | ||||||||
| Non-smoker (Ref) | ||||||||
| Current smoker | −1.96 (−2.47, −1.45) | <0.0001 | −2.11 (−2.66, −1.55) | <0.0001 | −2.94 (−3.91, −1.98) | <0.0001 | −2.52 (−3.46, −1.57) | <0.0001 |
| Past smoker | −0.15 (−0.60, 0.29) | 0.5035 | −0.26 (−0.75, 0.23) | 0.2963 | −0.96 (−1.90, −0.02) | 0.0457 | −1.09 (−1.99,−0.19) | 0.0181 |
Acronyms: LMM: Linear Mixed-Effects Models; BMI: Body Mass Index; PGS: Polygenic Risk Score; GED: General Educational Development/Certificate of High School Equivalency; CESD: Center for Epidemiologic Studies Depression Scale, 8-item; SES: Socioeconomic Status; CI: Confidence Interval.
To illustrate interactions between PGS and obesogenic factors on BMI, we present the difference in mean BMI between the 10th and 90th PGS percentiles (i.e., PGS=0.1 and PGS=0.9 in Figure 2 and Table 3 for PGS and age interaction, and supplementary Table S3 for PGS interactions with physical activity, alcohol intake, and cSES).
Figure 2.

Estimated mean BMI trajectories from 50 to 70 years of age and 95% confidence bands evaluated at 10th and 90th PGS percentiles for White and Black men and women study participants. PGS-by-Age interactions were observed for White men: Pinteraction = 0.0383, White women: Pinteraction = 0.0514, Black men: Pinteraction = 0.9496, White women: Pinteraction = 0.1883.
Table 3.
Estimated mean BMI at the 10th and 90th PGS percentiles (PGS=0.1 and PGS=0.9) at age 50, 60, 70; difference and relative difference in mean BMI between PGS percentiles (PGS effect) at age 50, 60, 70, and the changes in PGS effect at age 60 and 70 relative to age 50.
| Direct and Relative Difference in BMI between PGS (PGS Effect) | Changes in PGS Effect Relative to Age 50 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | PGS | (95% CI) | BMIq0.9-BMIq0.1 | p-value | BMIq0.9/BMI0.1 | p-value | Direct Change | p-value | Relative Change | p-value |
| White Men (Pinteraction=0.0383) | ||||||||||
| 50 | 0.1 | 27.22 (26.34, 28.09) | ||||||||
| 0.9 | 31.47 (30.68, 32.26) | 4.25 (2.97, 5.81) | <0.0001 | 1.16 (1.11, 1.21) | <0.0001 | Reference Age | Reference Age | |||
| 60 | 0.1 | 28.03 (27.65, 28.41) | ||||||||
| 0.9 | 31.94 (31.56, 32.32) | 3.91 (3.27, 4.56) | <0.0001 | 1.14 (1.11, 1.17) | <0.0001 | −0.34 (−1.98, 1.02) | 0.5334 | 0.92 (0.58, 1.26) | 0.6289 | |
| 70 | 0.1 | 28.79 (28.29, 29.30) | ||||||||
| 0.9 | 31.90 (31.38, 32.42) | 3.11 (2.19, 4.04) | <0.0001 | 1.11 (1.08, 1.14) | <0.0001 | −1.14 (−2.82, 0.27) | 0.1061 | 0.73 (0.43, 1.03) | 0.0734 | |
| White Women (Pinteraction=0.0514) | ||||||||||
| 50 | 0.1 | 25.99 (25.22, 26.76) | ||||||||
| 0.9 | 32.81 (32.03, 33.59) | 6.82 (5.62, 7.99) | <0.0001 | 1.26 (1.19, 1.25) | <0.0001 | Reference Age | Reference Age | |||
| 60 | 0.1 | 27.20 (26.77, 27.63) | ||||||||
| 0.9 | 33.21 (32.78, 33.64) | 6.01 (5.32, 6.68) | <0.0001 | 1.22 (1.19, 1.25) | <0.0001 | −0.81 (−1.97, 0.36) | 0.175 | 0.88 (0.73, 1.05) | 0.1665 | |
| 70 | 0.1 | 27.35 (26.81, 27.88) | ||||||||
| 0.9 | 32.83 (32.28, 33.37) | 5.48 (4.57, 6.35) | <0.0001 | 1.20 (1.16, 1.24) | <0.0001 | −1.34 (−2.60, −0.09) | 0.0358 | 0.80 (0.64, 0.97) | 0.023 | |
| Black Men (Pinteraction=0.9496) | ||||||||||
| 50 | 0.1 | 27.36 (26.27, 28.46) | ||||||||
| 0.9 | 30.72 (29.62, 31.81) | 3.35 (1.55, 5.14) | 0.0003 | 1.12 (1.05, 1.20) | 0.0009 | Reference Age | Reference Age | |||
| 60 | 0.1 | 27.48 (26.65, 28.31) | ||||||||
| 0.9 | 30.87 (30.05, 31.69) | 3.39 (2.00, 4.81) | <0.0001 | 1.12 (1.07, 1.18) | <0.0001 | −0.04 (−1.10, 1.21) | 0.9244 | 1.01 (0.54, 1.59) | 0.8129 | |
| 70 | 0.1 | 27.59 (26.40, 28.78) | ||||||||
| 0.9 | 31.02 (29.87, 32.17) | 3.43 (1.61, 5.31) | 0.0002 | 1.12 (1.06, 1.20) | 0.0005 | 0.08 (−2.20, 2.42) | 0.9244 | 1.02 (0.08, 2.18) | 0.8128 | |
| Black Women (Pinteraction=0.1883) | ||||||||||
| 50 | 0.1 | 31.95 (30.86, 33.04) | ||||||||
| 0.9 | 35.01 (33.94, 36.09) | 3.06 (0.99, 4.67) | 0.0025 | 1.10 (1.03, 1.15) | 0.0029 | Reference Age | Reference Age | |||
| 60 | 0.1 | 31.91 (31.06, 32.76) | ||||||||
| 0.9 | 34.21 (33.36, 35.05) | 2.30 (0.65, 3.51) | 0.0045 | 1.07 (1.02, 1.11) | 0.0054 | −0.64 (−1.98, 0.48) | 0.2296 | 0.75 (0.48, 2.00) | 0.7038 | |
| 70 | 0.1 | 31.86 (30.73, 33.00) | ||||||||
| 0.9 | 33.40 (32.27, 34.54) | 1.54 (−0.61, 3.26) | 0.1804 | 1.05 (0.98, 1.10) | 0.1851 | 1.52 (−3.97, 0.95) | 0.2296 | 0.50 (−1.95, 3.00) | 0.702 | |
Acronyms: BMI: Body Mass Index; PGS: Polygenic Risk Score
PGS-Age Interaction
Interactions between PGS and age were observed among White men (Pinteraction=0.0383) and women (Pinteraction=0.0514); the influence of PGS attenuated with older age (Figure 2; Table 3). Among White men, at age 50, the mean BMI difference between the 90th and 10th percentiles was 4.25 kg/m2 (95%CI: 2.97, 5.81; p<0.0001). At age 70, the mean BMI difference was 3.11 kg/m2 (95%CI: 2.19, 4.04; p<0.0001), which is 73% that of age 50 (or 1.14 kg/m2 lower BMI). Similarly, among White women aged 50 years, the mean BMI difference between the 90th and 10th PGS percentiles were 6.82 kg/m2 (95%CI: 5.62, 7.99; p<0.0001), compared to 5.48 kg/m2 (95% CI: 4.57, 6.35; p<0.0001) at age 70 (80% that of age 50 or 1.34 kg/m2 less). No significant PGS-age interaction was detected among Black men (Pinteraction=0.9496) or women (Pinteraction=0.1883).
PGS-Physical Activity Interaction
Physical activity appeared to modify the effect of PGS on BMI among White women (Figure 3a). Vigorous physical activity was associated with lower mean BMI compared to those reporting some/no physical activity. The protective effect of vigorous physical activity on BMI was stronger with increasing PGS (=−1.04 kg/m2, 95%CI: −2.10, 0.02, p=0.0546). For women in the 90th PGS percentile, vigorous physical activity was associated with 1.66 kg/m2 (95%CI: 1.06, 2.29; p<0.0001) lower mean BMI compared to those reporting some/no physical activity, which was twice that of the 10th PGS percentile: 0.83 kg/m2 (95%CI: 0.37, 1.29; p=0.0005). A similar pattern was observed with regards to moderate physical activity, compared to some/never, as well as for White men; however, these interactions were not statistically significant.
Figure 3.
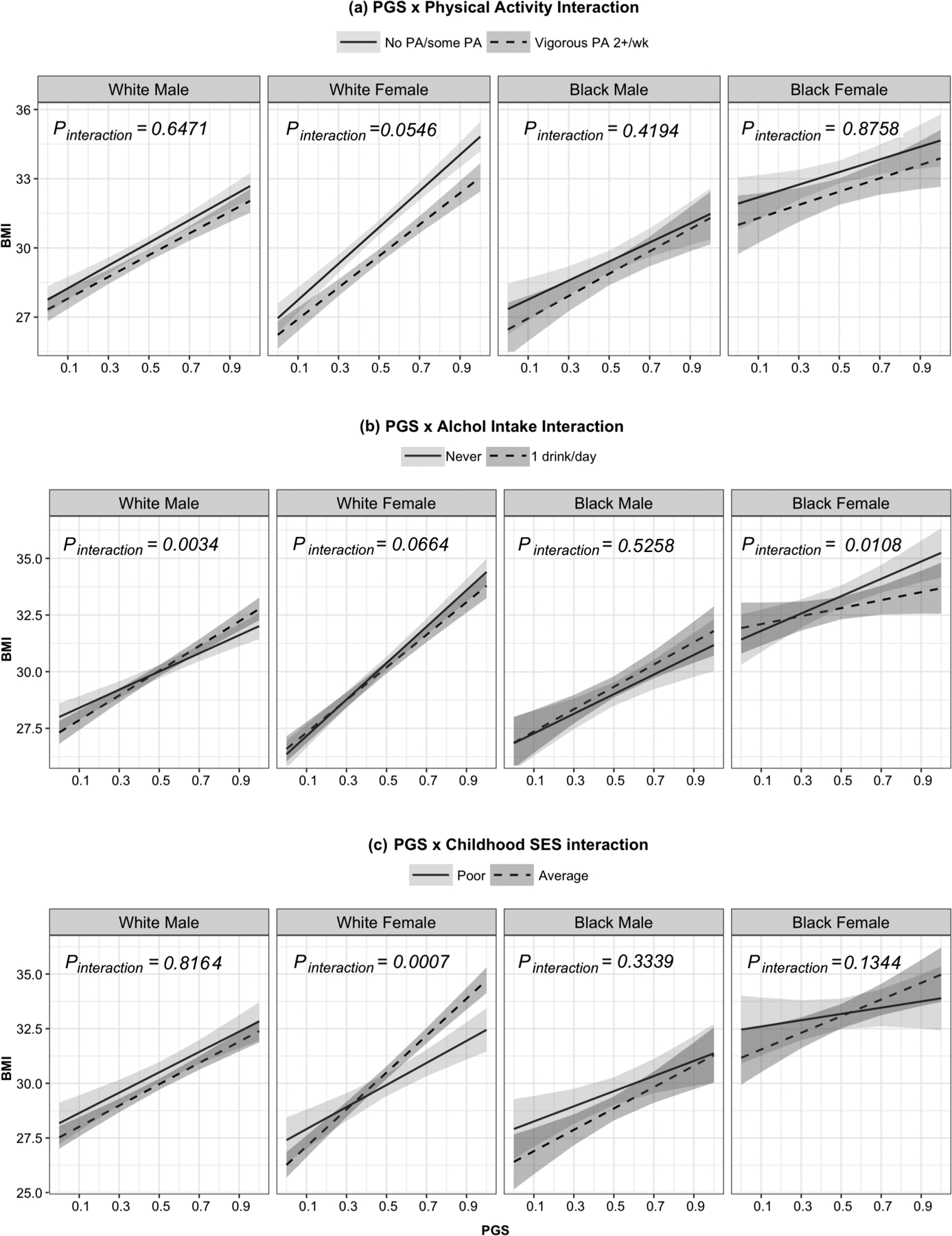
Interaction between PGS and (a) vigorous activities versus no/some physical activity, (b) 1 alcoholic drink per day versus none, and (c) poor childhood SES versus average. The shaded areas are the 95% confidence bands for estimated mean BMI. (See supplementary Table S3 for the estimated difference between the 10th and 90th PGS percentile and Figure S1 for all levels for each variable).
PGS-Alcohol Consumption & PGS-Childhood Socioeconomic Status Interactions
Interaction was observed between PGS and low levels of alcohol intake (Figure 3b) among White men and women and Black women, but with different directions in men and women. Among women, consuming an average of one alcoholic drink per day, versus none, was associated with slower rate of increase in BMI induced by PGS (White women: =−0.85, 95%CI: −1.75, 0.06, p=0.0664; Black women: =−2.07, 95%CI: −3.65, −0.48, p=0.0108). Higher rates of change in BMI by PGS were found in men (White men: =1.44, 95%CI: 0.48, 2.41, p=0.0034; Black men: =0.60, 95%CI: −1.24, 2.44, p=0.5258).
Interaction between cSES and PGS on BMI was observed only among White women. For lower PGS percentiles, the mean BMI among average cSES White women was lower than women with poor cSES (Figure 3c). However, the rate of change in BMI by PGS was greater in average cSES White women (3.40 kg/m2, 95%CI: 1.43, 5.38, Pinteraction=0.0007). The difference in mean BMI at the 10th PGS percentile −0.79 kg/m2 and 1.93 kg/m2 in the 90th percentile. A similar, but not statistically significant, pattern was observed in Black women.
Discussion
Our study examined the longitudinal interplay of polygenic risk and obesogenic characteristics, including demographic and modifiable lifestyle factors, on objectively measured BMI within two nationally representative samples of older Black and White men and women from the HRS. We first demonstrated evidence for the BMI-elevating effect of underlying polygenic risk, derived from the recent Locke et al.37 meta-analysis, and the differential rank percentile effects of PGS across racial- and sex-specific subgroups on average BMI. Higher PGS percentiles appeared to have a greater BMI-elevating effect among White participants, compared to Black participants, particularly women. These findings may indicate that the PGS, derived primarily from participants of European descent37, does not adequately represent the underlying genetic determinants of BMI among Black Americans.
We observed significant interactions between PGS and several demographic and behavioral factors. In our adjusted analyses, we observed that the association between elevated polygenic risk and BMI attenuated by increasing age. Walter et al.25 reported a similar birth cohort interaction among HRS participants utilizing a 29-SNP PGS and self-reported BMI; that is, the association between PGS and self-reported BMI was greater among White participants from younger cohorts, suggesting that genetic risk becomes less influential in older adulthood. Aside from the updated PGS, versus the aforementioned 29-SNP PGS, our study differs from Walter et al.25 by accounting for several obesogenic environmental factors, including physical activity, depression, alcohol consumption, and smoking; however, changes to other environmental factors that contribute to the age-related effects may explain the age-PGS interaction, particularly differences in unmeasured dietary behaviors between younger and older adults. A growing number of gene-environment studies on BMI show a significant interaction between dietary factors and genetic risk in adults.13, 42–45 However, this hypothesis does not sufficiently explain why the age-PGS interaction was only detected among White, and not Black, participants, since dietary shifts often disproportionately affect BMI variation among ethnic minority groups.46
The effect of BMI PGS was stronger among White women reporting some or no physical activity compared to those reporting vigorous physical activity twice or more per week. Tyrrell et al.21 demonstrated similar findings utilizing a 69-SNP PGS derived from the UK Biobank study, where the effect of vigorous activity (>1 hour compared to ≤ 1 hour weekly) was stronger in the 10% highest genetic risk than those in the 10% lowest genetic risk. Our interaction results were consistent with these findings among White women (p=0.055), but not among White men (p=0.647). Moreover, a similar interaction effect was observed in postmenopausal women of European ancestry from the Women’s Health Initiative.12 Thus, our findings replicated those of prior studies suggesting that vigorous physical activity may mitigate the effects of genetic predisposition to obesity, and suggest that these findings may be stronger among White women.
Among White men, the BMI-elevating association of PGS was significantly stronger among those reporting one alcoholic drink per day versus alcohol abstinence. These findings were unexpected as several recent gene-environment studies observe a significant attenuation of PGS for BMI higher alcohol consumption compared to abstainers.20, 23, 27 However, low-level alcohol use appeared to significantly attenuate the PGS effect among women in a similar manner reported in the literature. Moreover, we observed that higher alcohol consumption had a similar effect on mean BMI as alcohol abstinence across PGS levels in While men. One explanation may be that alcohol abstainers previously drank heavily and quit prior to the baseline questionnaire. Similarly, average cSES was associated with a significantly higher BMI among White women with the highest PGS, relative to both poor and well-off cSES; however, average cSES was associated with lower BMI among those with the lowest PGS. Given the well-documented association between adverse childhood experiences, including financial difficulties, and premature mortality,47, 48 differing survival prior to cohort entry may explain the greater PGS effect among those with average cSES. Nonetheless, while we offer some explanations for these differences, it is important to note that the proportion of individuals within higher levels of daily alcohol use and well-off cSES are markedly small; thus, interpretation of these patterns should be made cautiously. Further investigation is warranted to elucidate the interaction between alcohol use, cSES, and polygenic risk.
There are several important limitations to consider when interpreting our findings. First, the SNPs used in calculating the PGS derive largely from GWAS of European groups, and thus, may not encapsulate the underlying genetic risk among not only Black American, but White Americans, as well. Moreover, while HRS uses a relatively recent GWAS meta-analysis from the GIANT Consortium to calculate PGS, a larger and more recently published GWAS meta-analysis using discovery data from the UK Biobank and the GIANT Consortium identified 941 near-independent SNPs explaining approximately 6.0% of the variance of BMI in the HRS replication sample.49 Without access to the HRS GWAS data, the PGS we use is limited to the most recent version produced by the HRS team; however, the correlation between PGS and BMI in HRS participants is near identical to the correlation between the PGS derived from the latest meta-analysis (r = 0.220) and the PGS used in our study (r = 0.257 for White participants, and r = 0.216 for both Black and White participants combined).49 Second, our use of measured BMI led to a relatively small sample size of HRS participants, possibly impeding our ability to detect other important main and interaction effects. Third, in using an older cohort of adults, survival bias may have led to a sample of healthier participants, compared to the general U.S. population.30 Survival bias may have disproportionately affected Black participants prior to cohort entry.50 Fourthly, the self-reported behaviors are subject to information biases; however, it is unlikely that social desirability would differentially affect responses by BMI or genetic variation as both were objectively measured. Lastly, BMI does not fully encapsulate adiposity, as it does not account for the various components of body composition.51 This issue may be particularly problematic given our sample of older adults, as decreases in BMI may be attributed to age-related muscle loss, contributing to adverse health outcomes.52
Despite these limitations, our study had many strengths. To our knowledge, we are the first to quantify both the independent effect of PGS, as well as the interactions between PGS and lifestyle factors, among sex- and ethnic-specific strata from a nationally representative sample of older adults. Quantifying these associations within different groups offers crucial insights that may improve precision in therapeutic approaches targeting BMI in older adulthood. For instance, examining the determinants of sex-linked biological traits, such as BMI, may contribute crucial information on how differences in health outcomes may occur.53 Moreover, while other studies have examined similar covariate interaction in other cohorts, our study is distinguished by utilizing a PGS incorporating a more recent GWAS meta-analysis, thus providing a more robust measurement of underlying genetic risk.37 In addition, while many gene-environment studies on BMI use self-reported BMI, our use of objectively-measured BMI greatly reduces the possibility of a differential misclassification in our outcome measure. Therefore, despite our relatively modest sample size, there is substantial reliability in the observed interactions between PGS and obesogenic environmental factors across sex and race.
In conclusion, we observed significant associations between PGS and obesogenic environment factors on BMI among White men and women from a nationally representative sample of older adults. Future investigations may benefit from examining interaction between genetic risk and changing dietary trends, the latter of which is contributing to recent, secular elevations in BMI. Moreover, further inquiry into additional genetic loci among non-Europeans may further explain the distinct pattern of association between PGS and BMI we observed among Black participants. Researchers should continue to focus on understanding the complex interplay between genetics and environmental factors on BMI, particularly among understudied, underserved, and high-risk populations, to better inform understanding of the underlying etiology of obesity and how to intervene.
Supplementary Material
Acknowledgments
This study was supported by Ola HAWAII through the National Institute on Minority Health and Health Disparities (U54MD007601-31), National Institutes of Health. We declare that we have no conflicts of interest in the authorship or publication of this contribution.
References
- 1.Boles A, Kandimalla R, Reddy PH. Dynamics of diabetes and obesity: Epidemiological perspective. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2017; 1863(5): 1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastien M, Poirier P, Lemieux I, Després J-P. Overview of Epidemiology and Contribution of Obesity to Cardiovascular Disease. Progress in Cardiovascular Diseases 2014; 56(4): 369–381. [DOI] [PubMed] [Google Scholar]
- 3.Arnold M, Leitzmann M, Freisling H, Bray F, Romieu I, Renehan A et al. Obesity and cancer: An update of the global impact. Cancer Epidemiology 2016; 41: 8–15. [DOI] [PubMed] [Google Scholar]
- 4.Bhaskaran K, Dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3.6 million adults in the UK. The lancet. Diabetes & endocrinology 2018; 6(12): 944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tremmel M, Gerdtham U-G, Nilsson P, Saha S. Economic Burden of Obesity: A Systematic Literature Review. International Journal of Environmental Research and Public Health 2017; 14(4): 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA 2013; 309(1): 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the united states, 2005 to 2014. JAMA 2016; 315(21): 2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong SS, Zhang YJ, Chen YX, Yao S, Hao RH, Rong Y et al. Comprehensive review and annotation of susceptibility SNPs associated with obesity‐related traits. Obesity Reviews 2018. [DOI] [PubMed] [Google Scholar]
- 9.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015; 518(7538): 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Speakman JR, Loos RJF, O’Rahilly S, Hirschhorn JN, Allison DB. GWAS for BMI: a treasure trove of fundamental insights into the genetic basis of obesity. International journal of obesity (2005) 2018; 42(8): 1524–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Celis-Morales CA, Lyall DM, Bailey MES, Petermann-Rocha F, Anderson J, Ward J et al. The Combination of Physical Activity and Sedentary Behaviors Modifies the Genetic Predisposition to Obesity. Obesity 2019; 27(4): 653–661. [DOI] [PubMed] [Google Scholar]
- 12.Ochs-Balcom HM, Preus L, Nie J, Wactawski-Wende J, Agyemang L, Neuhouser ML et al. Physical activity modifies genetic susceptibility to obesity in postmenopausal women. Menopause-J. N. Am. Menopause Soc 2018; 25(10): 1131–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang TG, Heianza Y, Sun DJY, Huang T, Ma WJ, Rimm EB et al. Improving adherence to healthy dietary patterns, genetic risk, and long term weight gain: gene-diet interaction analysis in two prospective cohort studies. BMJ-British Medical Journal 2018; 360: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svendstrup M, Allin KH, Sorensen TIA, Hansen TH, Grarup N, Hansen T et al. Genetic risk scores for body fat distribution attenuate weight loss in women during dietary intervention. Int. J. Obes 2018; 42(3): 370–375. [DOI] [PubMed] [Google Scholar]
- 15.Ding M, Ellervik C, Huang T, Jensen MK, Curhan GC, Pasquale LR et al. Diet quality and genetic association with body mass index: results from 3 observational studies. Am. J. Clin. Nutr 2018; 108(6): 1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celis-Morales C, Lyall DM, Guo YB, Steell L, Llanas D, Ward J et al. Sleep characteristics modify the association of genetic predisposition with obesity and anthropometric measurements in 119,679 UK Biobank participants. Am. J. Clin. Nutr 2017; 105(4): 980–990. [DOI] [PubMed] [Google Scholar]
- 17.Llewellyn C, Wardle J. Behavioral susceptibility to obesity: Gene–environment interplay in the development of weight. Physiology & Behavior 2015; 152: 494–501. [DOI] [PubMed] [Google Scholar]
- 18.Wang NJ, Lu M, Chen C, Xia FZ, Han B, Li Q et al. Adiposity Genetic Risk Score Modifies the Association Between Blood Lead Level and Body Mass Index. J. Clin. Endocrinol. Metab 2018; 103(11): 4005–4013. [DOI] [PubMed] [Google Scholar]
- 19.Nagpal S, Gibson G, Marigorta UM. Pervasive Modulation of Obesity Risk by the Environment and Genomic Background. Genes 2018; 9(8): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rask-Andersen M, Karlsson T, Ek WE, Johansson A. Gene-environment interaction study for BMI reveals interactions between genetic factors and physical activity, alcohol consumption and socioeconomic status. PLoS Genet. 2017; 13(9): 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyrrell J, Wood AR, Ames RM, Yaghootkar H, Beaumont RN, Jones SE et al. Gene-obesogenic environment interactions in the UK Biobank study. Int. J. Epidemiol 2017; 46(2): 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song M, Zheng Y, Qi L, Hu FB, Chan AT, Giovannucci EL. Longitudinal Analysis of Genetic Susceptibility and BMI Throughout Adult Life. Diabetes 2018; 67(2): 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohde JF, Angquist L, Larsen SC, Tolstrup JS, Husemoen LLN, Linneberg A et al. Alcohol consumption and its interaction with adiposity-associated genetic variants in relation to subsequent changes in waist circumference and body weight. Nutr. J 2017; 16: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stryjecki C, Alyass A, Meyre D. Ethnic and population differences in the genetic predisposition to human obesity. Obesity Reviews 2018; 19(1): 62–80. [DOI] [PubMed] [Google Scholar]
- 25.Walter S, Mejia-Guevara I, Estrada K, Liu SY, Glymour MM. Association of a Genetic Risk Score With Body Mass Index Across Different Birth Cohorts. JAMA-J. Am. Med. Assoc 2016; 316(1): 63–69. [DOI] [PubMed] [Google Scholar]
- 26.Zhao W, Ware E, He Z, Kardia S, Faul J, Smith J. Interaction between social/psychosocial factors and genetic variants on body mass index: A gene-environment interaction analysis in a longitudinal setting. International journal of environmental research and public health 2017; 14(10): 1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calvin CM, Hagenaars SP, Gallacher J, Harris SE, Davies G, Liewald DC et al. Sex-specific moderation by lifestyle and psychosocial factors on the genetic contributions to adiposity in 112,151 individuals from UK Biobank. Sci Rep 2019; 9: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher GG, Ryan LH. Overview of the Health and Retirement Study and Introduction to the Special Issue. Work, aging and retirement 2018; 4(1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort Profile: the Health and Retirement Study (HRS). Int J Epidemiol 2014; 43(2): 576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domingue BW, Belsky DW, Harrati A, Conley D, Weir DR, Boardman JD. Mortality selection in a genetic sample and implications for association studies. Int J Epidemiol 2017; 46(4): 1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steffick DE. Documentation of affective functioning measures in the Health and Retirement Study Ann Arbor, MI: University of Michigan; 2000. [Google Scholar]
- 32.Drewnowski A, Specter SE. Poverty and obesity: the role of energy density and energy costs. Am J Clin Nutr 2004; 79(1): 6–16. [DOI] [PubMed] [Google Scholar]
- 33.Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BWJH et al. Overweight, Obesity, and Depression: A Systematic Review and Meta-analysis of Longitudinal Studies. JAMA Psychiatry 2010; 67(3): 220–229. [DOI] [PubMed] [Google Scholar]
- 34.Clarke TK, Hall LS, Fernandez-Pujals AM, MacIntyre DJ, Thomson P, Hayward C et al. Major depressive disorder and current psychological distress moderate the effect of polygenic risk for obesity on body mass index. Transl. Psychiatr 2015; 5(6): e592–e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faith MS, Kral TVE. Social Environmental and Genetic Influences on Obesity and Obesity-Promoting Behaviors: Fostering Research Integration In: Hernandez LM, Blazer DG (eds). Institute of Medicine (US) Committee on Assessing Interactions Among Social, Behavioral, and Genetic Factors in Health. National Academies Press (US): Washington, DC, 2006. [Google Scholar]
- 36.Johnson W, Ong KK, Elks CE, Wareham NJ, Wong A, Muniz-Terrera G et al. Modification of genetic influences on adiposity between 36 and 63 years of age by physical activity and smoking in the 1946 British Birth Cohort Study. Nutrition & Diabetes 2014; 4: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015; 518(7538): 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ware E, Schmitz L, Gard A, Faul J. HRS polygenic scores—Release 3: 2006–2012 genetic data. Ann Arbor: Survey Research Center, University of Michigan 2018. [Google Scholar]
- 39.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis, vol. 998 John Wiley & Sons, 2012. [Google Scholar]
- 40.McDonald L. Tests for the general linear hypothesis under the multiple design multivariate linear model. The Annals of Statistics 1975; 3(2): 461–466. [Google Scholar]
- 41.Efron B, Tibshirani RJ. An introduction to the bootstrap, CRC press, 1994. [Google Scholar]
- 42.Hosseini-Esfahani F, Koochakpoor G, Daneshpour MS, Sedaghati-khayat B, Mirmiran P, Azizi F. Mediterranean Dietary Pattern Adherence Modify the Association between FTO Genetic Variations and Obesity Phenotypes. Nutrients 2017; 9(10): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brunkwall L, Chen Y, Hindy G, Rukh G, Ericson U, Barroso I et al. Sugar-sweetened beverage consumption and genetic predisposition to obesity in 2 Swedish cohorts. Am. J. Clin. Nutr 2016; 104(3): 809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casas-Agustench P, Arnett DK, Smith CE, Lai CQ, Parnell LD, Borecki IB et al. Saturated Fat Intake Modulates the Association between an Obesity Genetic Risk Score and Body Mass Index in Two US Populations. J. Acad. Nutr. Diet 2014; 114(12): 1954–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goni L, Cuervo M, Milagro FI, Martinez JA. A genetic risk tool for obesity predisposition assessment and personalized nutrition implementation based on macronutrient intake. Genes Nutr. 2015; 10(1): 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gilbert PA, Khokhar S. Changing dietary habits of ethnic groups in Europe and implications for health. Nutrition reviews 2008; 66(4): 203–215. [DOI] [PubMed] [Google Scholar]
- 47.Kelly-Irving M, Lepage B, Dedieu D, Bartley M, Blane D, Grosclaude P et al. Adverse childhood experiences and premature all-cause mortality. Eur J Epidemiol 2013; 28(9): 721–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown DW, Anda RF, Tiemeier H, Felitti VJ, Edwards VJ, Croft JB et al. Adverse childhood experiences and the risk of premature mortality. American journal of preventive medicine 2009; 37(5): 389–96. [DOI] [PubMed] [Google Scholar]
- 49.Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN et al. Meta-analysis of genome-wide association studies for height and body mass index in approximately 700000 individuals of European ancestry. Hum Mol Genet 2018; 27(20): 3641–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shuey KM, Willson AE. Cumulative Disadvantage and Black-White Disparities in Life-Course Health Trajectories. Research on Aging 2008; 30(2): 200–225. [Google Scholar]
- 51.Rothman KJ. BMI-related errors in the measurement of obesity. Int. J. Obes 2008; 32: S56. [DOI] [PubMed] [Google Scholar]
- 52.Ferrucci L, Cooper R, Shardell M, Simonsick EM, Schrack JA, Kuh D. Age-Related Change in Mobility: Perspectives From Life Course Epidemiology and Geroscience. The journals of gerontology. Series A, Biological sciences and medical sciences 2016; 71(9): 1184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krieger N. Genders, sexes, and health: what are the connections—and why does it matter? Int. J. Epidemiol 2003; 32(4): 652–657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


