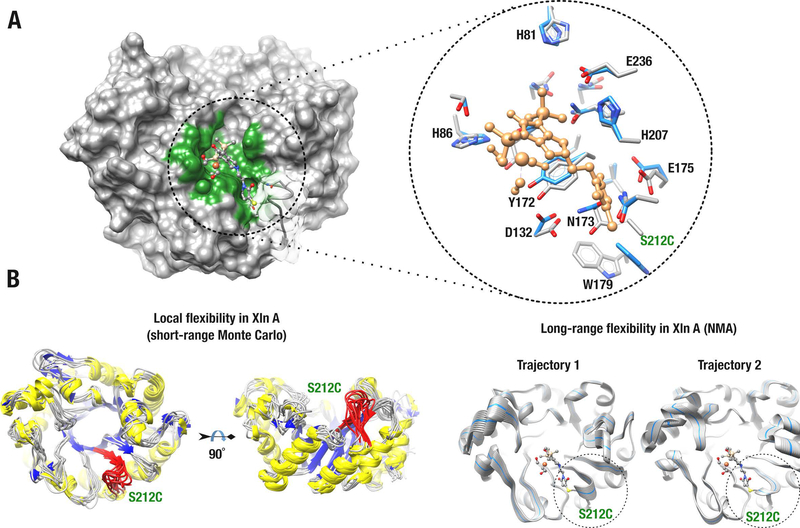
Figure 2.
Biohybrid model of the Knölker’s complex covalently anchored to Xln A. A) Energy-minimized structure of Xln A covalently bound to the Knölker’s complex at mutated position C212. The left panel shows the surface of the Xln A active-site cavity (green) bound to the Knölker’s complex (ball-and-stick model). The right panel shows residue side-chains that stabilize the Knölker’s complex in the active-site cavity of Xln A. Surface transparency was applied to illustrate the active-site loop harboring residue C212. Residue side chains are labeled and shown before (blue) and after (gray) energy minimization. The Knölker’s complex is shown in sandy brown and the C212 substitution is labeled in green. B) Molecular dynamics simulations illustrating short-range (left panel) and long-range (right panel) conformational exchange experienced by Xln A. The C212 loop is colored red (left) and highlighted by a dashed circle (right). For long-range trajectory simulations, the starting Xln A conformer is shown as a blue ribbon.