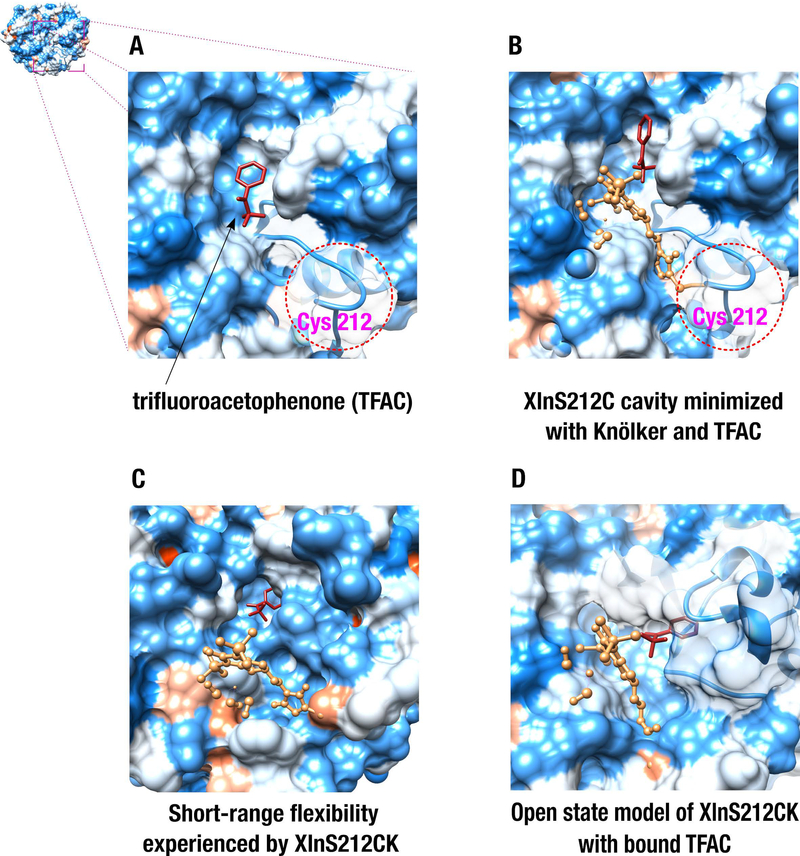
Figure 6.
Virtual docking of the trifluoroacetophenone (TFAC) substrate to the XlnS212C and XlnS212CK biohybrid complexes used as receptor targets. A) Blind virtual docking of TFAC to the XlnS212C variant obtained from the RosettaBackrub server. B) Docking of TFAC to the energetically minimized cavity of the XlnS212CK biohybrid complex. C) Docking of TFAC to an energetically favorable XlnS212CK conformer extracted from Monte Carlo simulations. D) Virtual docking of TFAC to the open state conformation of XlnS212CK, obtained from Normal Mode Analysis. In all structures, TFAC and the Knölker’s complex are colored red and sandy brown, respectively. Red and blue protein surfaces highlight hydrophobic and hydrophilic surface areas, respectively.