Figure 1: ANT protein schematic.
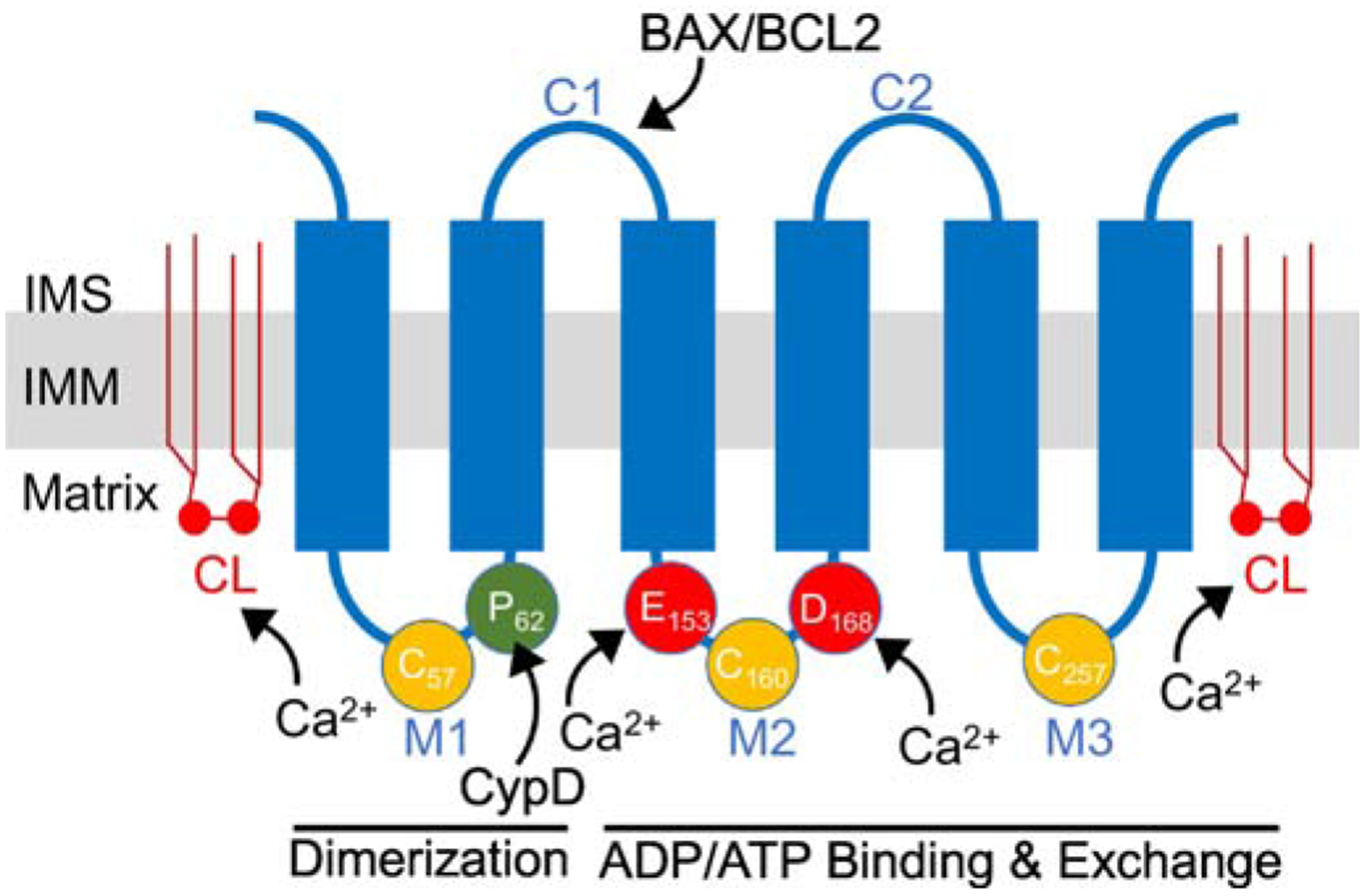
Schematic of ANT protein monomer topology in the inner mitochondrial membrane (IMM). ANT proteins have six transmembrane domains and two mitochondrial intermembrane (IMS) space facing domains (C1-C2). C1 contains a BAX/BCL2 binding motif. In addition, there are three mitochondrial matrix facing loops (M1-M3). The M1 loop is thought to be involved in dimerization and it contains Proline-62 (P62) that is thought to interact with CypD. This M1 loop also contains Cysteine-57 (C57) that is thought to play a role in ANT dimerization. The M2 and M3 loops are involved in ADP/ATP binding and nucleotide exchange. M2 and M3 contain two modifiable cysteines (C160 and C257) that are thought to be sensitive to oxidative stress. In addition, M2 contains two acidic amino acids, Gultamate-153 (E153) and Asparatate-168 (D168), important to ADP binding that may also be involved in Ca2+ sensing and regulation. In addition, ANT proteins are associated with six molecules of cardiolipin (depicted by CL) in the inner membrane that is required for protein folding and sensitivity to Ca2+ regulation. Amino acid residue numbers are taken from human ANT1.
