Figure 1.
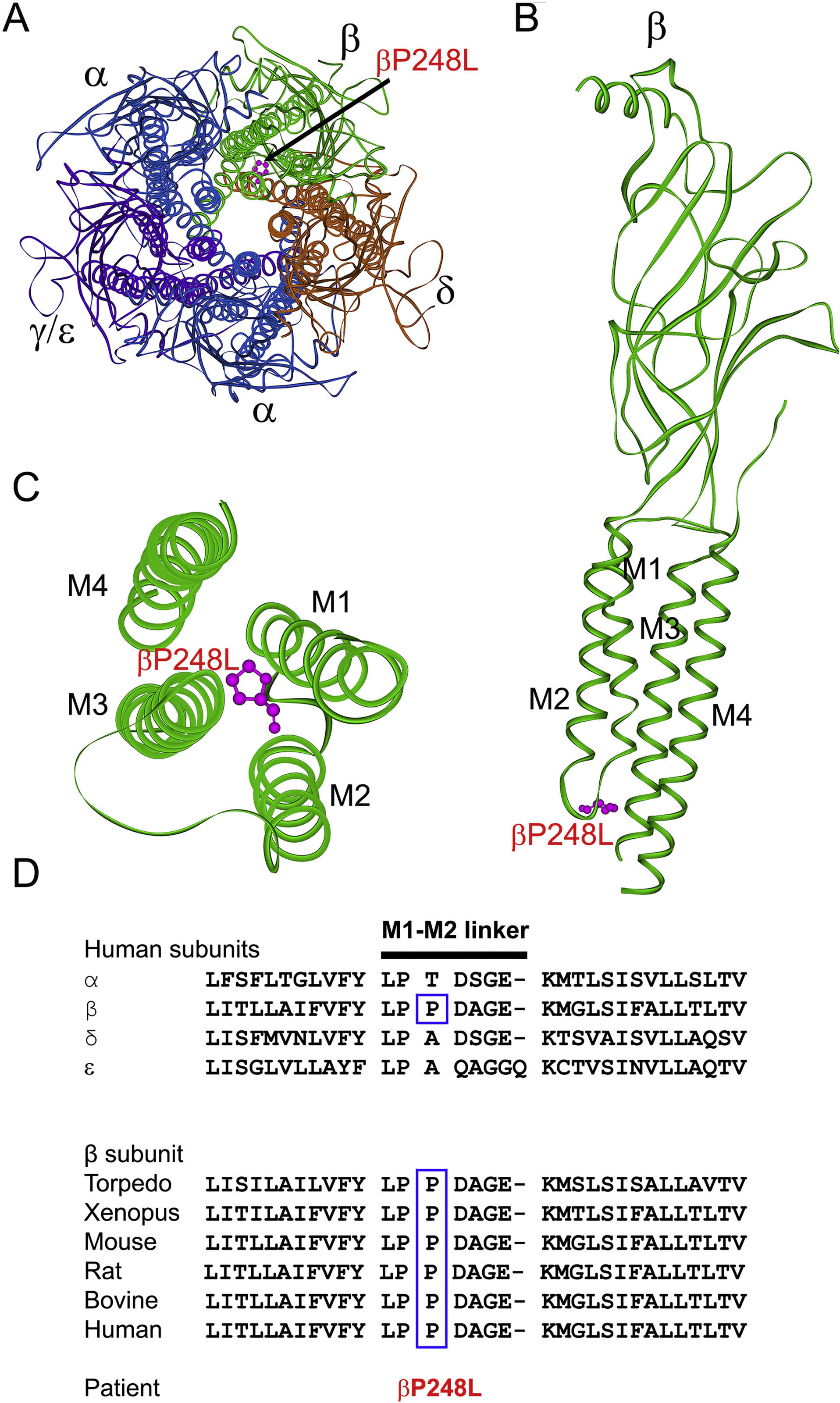
(A) Structure the AChR viewed from the synaptic space indicating the location of the mutant P248 residue (pink) in the M1-M2 linker of the β subunit based on the α-bugarotoxin-bound structure of native Torpedo muscle AChR at a resolution of 2.7 Å (PDB# 6UWZ) (Rahman et al. 2020). (B) Side view of the extracellular and transmembrane domains of the β subunit. The MX helix stemming from the end of M3 is omitted for clarity. (C) Excellular view of the four transmembrane domains of the β subunit indicating the mutant residue βP248L in the M1-M2 linker projecting between the M3 and M4 helices. (D) Multiple sequence alignment of the AChR M1-M2 linkers. Proline at codon 248 in the human β subunit is conserved across all β subunits of different species.
