Abstract
Background
Adjuvant chemotherapy (AC) after esophagectomy improves survival in esophageal cancer when induction therapy has not been given; however, the optimal timing for initiation of AC is poorly characterized. We aimed to determine the impact of timing of AC on survival following esophagectomy.
Methods
The National Cancer Database (NCDB) was queried for patients with pT1–4aNxM0 esophageal cancer receiving AC with or without radiation from 2004–2015. The median and interquartile range of time to AC were determined. Patients were stratified by initiation of AC into four cohorts based on quartiles. Kaplan-Meier curves were generated and factors associated with survival were identified by Cox-proportional hazards modeling. A separate analysis was performed with time to AC as a continuous variable.
Results
A total of 1634 patients received AC following esophagectomy. Median time to receipt of AC was 59 days, with an interquartile range of 45–78 days. There was no significant difference in overall survival at five years (p=0.86) between groups. Median survival was 29 months in those receiving AC within 45 days and was 28 months in those receiving AC at other timepoints. On multivariable analysis, delay in receipt of AC beyond 45 days was not associated with inferior survival. This was preserved when time to AC was analyzed as a continuous variable (HR 1.0, 95% CI 1.0–1.0).
Conclusions
Timing of initiation of AC following esophagectomy does not appear to impact survival. Given the highly variable post-operative course following esophagectomy, the decision to start AC should involve multidisciplinary discussion and be made on a patient-by-patient basis.
Introduction
Surgery is the mainstay of curative treatment for localized and locally advanced esophageal cancer. Despite advances in minimally invasive surgery, esophagectomy remains a complex and morbid procedure, with mortality ranging from 8–23% and correlating with surgeon volume (1, 2). Postoperative recovery is often variable and lengthy, with malnutrition and deconditioning arising from extended hospital stays. The National Comprehensive Cancer Network (NCCN) guidelines recommend adjuvant chemotherapy with or without radiation for patients receiving upfront esophagectomy whose pathology demonstrates extensive local disease (pT2–4, node positive, or margin positive) (3). However, postoperative complications may delay the initiation of adjuvant therapy (4, 5).
The ideal timing between surgery and induction or adjuvant therapies is well studied in some malignancies (6–10). In patients with esophageal cancer who receive neoadjuvant therapy, numerous studies have examined this interval. Some studies have demonstrated that a longer interval between induction therapy and surgery is associated with a more complete pathologic response but worse postoperative morbidity and mortality, in addition to poorer long-term survival (11–14). However, literature is lacking on this relationship in patients receiving upfront esophagectomy followed by adjuvant therapy. Further, the NCCN guidelines do not specify an optimal timing for the initiation of adjuvant therapy (3).
Given the high-risk nature of esophagectomy and its associated morbidities, a patient’s desire and ability to receive adjuvant therapy and the timing at which it is initiated likely correlates with their recovery from surgery. Our study aims to explore contemporary practice patterns regarding the initiation of adjuvant chemotherapy following esophagectomy and its implications on survival in esophageal cancer, in order to help guide multidisciplinary decision making in the postoperative period.
Material and Methods
Data Source
The National Cancer Database (NCDB) is a nationwide clinical oncology database developed and maintained by the American Cancer Society and The Commission on Cancer (CoC) of the American College of Surgeons. Data ranging from demographics to treatment and outcomes is collected from over 1,500 CoC-accredited centers (15). Data is entered by trained cancer registrars using standardized definitions following extraction from medical records. Altogether, the NCDB is estimated to include more than 80% of new cancer diagnoses in the US (16), making it one of the most robust cancer databases available.
Study Design and Statistical Analysis
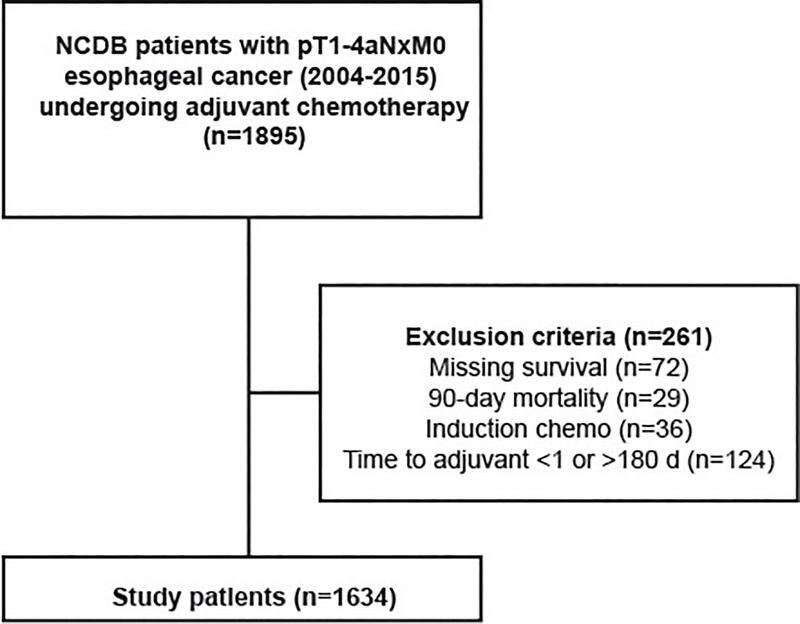
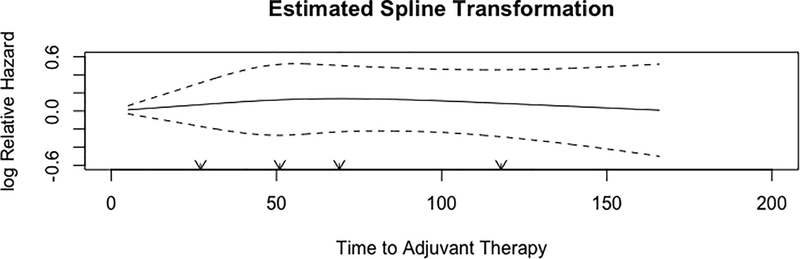
The NCDB was queried for patients with pT1–4aN0–3M0 adenocarcinoma or squamous cell carcinoma of the esophagus who underwent esophagectomy followed by adjuvant chemotherapy between 2004–2015. Patients with missing survival, receipt of neoadjuvant chemotherapy, 90-day postoperative mortality, and those starting adjuvant therapy greater than 6 months after surgery were excluded (Figure 1). Time from surgery to adjuvant therapy was plotted, and found to have a normal distribution. Time to adjuvant therapy was first modeled as a categorical variable based on distribution quartiles. In a separate analysis, time to adjuvant therapy was modeled as a continuous variable. A restricted cubic spline transformation, which utilizes cubic polynomial functions to approximate relationships, revealed that time to adjuvant therapy did not exhibit any demonstrable non-linear relationship with overall survival (Figure 2). As a result, time to adjuvant therapy was modeled as a continuous variable without the inclusion of spline functions.
Figure 1.
Study design (with inclusion and exclusion criteria) used to identify patients from the NCDB.
Figure 2.
Restricted cubic spline transformation of time to adjuvant therapy and impact on overall survival.
Baseline characteristics between the groups were compared using the Wilcoxon rank sum and Pearson’s chi-squared tests for continuous and categorical variables, respectively. The primary outcome was overall survival, which was estimated using Kaplan-Meier and Cox Proportional Hazards models. We selected variables for the Cox models a priori based on factors known to be associated with survival in patients undergoing esophagectomy, availability of these variables in the NCDB, and the effect of these variables on the time to adjuvant therapy variable. The fit of the model was evaluated using the likelihood ratio and the Akaike Information Criterion (AIC). Because of the number of patients in the cohort, parsimony was not a concern and all variables considered a priori were included in the Cox model. At least a 10:1 event to degree of freedom ratio was maintained in every regression performed. We used visual and quantitative assessments of Schoenfeld residuals to verify that the proportional hazards assumption was met for the variables included in the Cox model and the overall model itself. The Schoenfeld residual was nonsignificant (p>0.05) for each variable tested in every model. We tested age, pathologic stage, Charlson-Deyo comorbidity (CDCC) score, histology, length of stay, and 30-day unexpected readmission for interactions with the time to adjuvant therapy variable (Supplemental Table 1). A subgroup analysis was performed on patients with non-squamous pathology and lymph-node positive disease. Missing data were handled with complete case analysis. All statistical analyses were performed using R version 3.5.1 for Mac (Vienna, Austria). A two tailed p-value of less than or equal to 0.05 was considered statistically significant.
Results
Altogether, 1634 patients undergoing esophagectomy followed by adjuvant chemotherapy were identified (Figure 1). Time to adjuvant chemotherapy was normally distributed with a median of 59 days (IQR 45–78). In the first part of the study, patients were stratified approximately into quartiles for further analysis (Q1 d45 days, Q2 46–60 days, Q3 61–75 days, Q4 >75 days). Background characteristics are summarized in Table 1. Patients receiving adjuvant chemotherapy greater than 75 days after surgery were more likely to be older, black, have a later year of diagnosis, private insurance, squamous histology, and have had a longer hospital length of stay. Patients receiving adjuvant chemotherapy within 45 days of surgery were less likely to have received care at an academic or research institution. Further, there was a trend towards higher stage disease among those receiving therapy within 45 days. Overall, approximately 70% of patients received adjuvant radiation in addition to chemotherapy, with majority of these undergoing sequential therapy.
Table 1.
Demographic characteristics of study patients, stratified by time to adjuvant chemotherapy following esophagectomy.
| ≤45 Days (n=408)(%) | 46–60 Days (n=439)(%) | 61–75 Days (n=335)(%) | >75 Days (n=452)(%) | p-value | |
|---|---|---|---|---|---|
| Age (years, median) | 61 | 61 | 62 | 63 | 0.005 |
| Sex (female) | 53 (13) | 68 (16) | 52 (16) | 80 (18) | 0.30 |
| Race | 0.009 | ||||
| White | 386 (95) | 416 (96) | 310 (93) | 404 (90) | |
| Black | 13 (3) | 14 (3) | 14 (4) | 32 (7) | |
| Other | 6 (2) | 4 (1) | 8 (2) | 14 (3) | |
| Year of diagnosis, median (IQR) | 2007 (2005–2010) | 2007 (2005–2010) | 2007 (2005–2010) | 2008 (2006–2011) | <0.001 |
| Charlson-Deyo Comorbidity (CDCC) Score | 0.58 | ||||
| 0 | 306 (75) | 305 (70) | 245 (73) | 328 (73) | |
| 1 | 88 (22) | 109 (25) | 75 (22) | 100 (22) | |
| 2+ | 14 (3) | 25 (6) | 15 (5) | 24 (5) | |
| Insurance status | 0.02 | ||||
| Private | 225 (57) | 222 (51) | 161 (49) | 345 (80) | |
| Government | 164 (41) | 198 (46) | 167 (50) | 75 (17) | |
| None | 7 (2) | 13 (3) | 4 (1) | 11 (3) | |
| Facility location | 0.62 | ||||
| Metro | 323 (83) | 347 (82) | 259 (82) | 345 (80) | |
| Urban | 55 (14) | 71 (17) | 51 (16) | 75 (17) | |
| Rural | 11 (3) | 6 (1) | 5 (2) | 11 (3) | |
| Facility type | 0.006 | ||||
| Academic/research program | 138 (35) | 190 (45) | 153 (46) | 191 (42) | |
| Clinical stage | 0.48 | ||||
| 1 | 38 (19) | 47 (22) | 29 (20) | 55 (23) | |
| 2 | 61 (31) | 76 (36) | 57 (39) | 86 (35) | |
| 3 | 99 (50) | 91 (42) | 59 (41) | 191 (42) | |
| Pathologic stage | 0.12 | ||||
| 1 | 14 (4) | 8 (2) | 9 (3) | 8 (2) | |
| 2 | 141 (36) | 163 (39) | 140 (43) | 194 (44) | |
| 3 | 238 (61) | 247 (59) | 176 (54) | 236 (54) | |
| Margin status | 0.50 | ||||
| R0 | 310 (78) | 320 (75) | 268 (81) | 342 (79) | |
| R1 | 83 (21) | 104 (24) | 63 (19) | 90 (21) | |
| R2 | 2 (1) | 3 (1) | 1 (0) | 3 (0) | |
| Squamous histology | 40 (10) | 60 (14) | 42 (13) | 75 (17) | 0.03 |
| Number of adjuvant chemotherapy agents | 0.18 | ||||
| Single | 170 (31) | 175 (19) | 138 (37) | 194 (38) | |
| Multiple | 238 (69) | 264 (81) | 197 (63) | 258 (62) | |
| Perioperative radiation | 0.09 | ||||
| None | 116 (29) | 110 (25) | 93 (28) | 154 (34) | |
| Induction | 4 (1) | 3 (1) | 4 (1) | 2 (0.4) | |
| Adjuvant | 284 (70) | 324 (74) | 238 (71) | 293 (65) | |
| Sequence of adjuvant radiation and chemotherapy | 0.006 | ||||
| Concurrent | 86 (29) | 62 (19) | 43 (18) | 68 (22) | |
| Sequential | 202 (71) | 264 (81) | 195 (82) | 228 (78) | |
| Length of stay (median with IQR) | 9 (7–12) | 9 (7–12) | 10 (8–14) | 11 (8–18) | <0.001 |
| 30-day readmission | 27 (7) | 16 (4) | 20 (6) | 30 (7) | 0.18 |
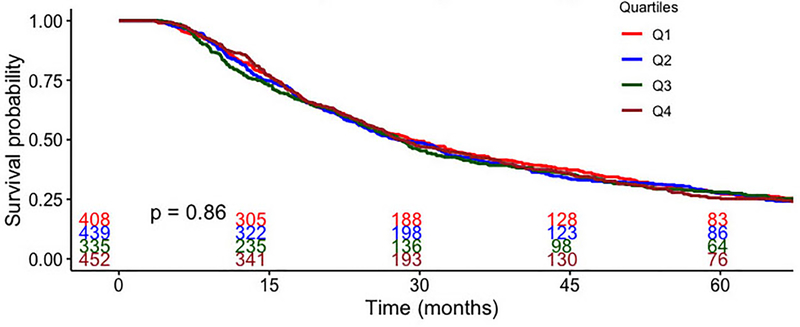
There was no significant difference in unadjusted overall survival at five years between groups (Figure 3). Median survival was 29 months for those receiving adjuvant chemotherapy within 45 days and was 28 months in those receiving adjuvant chemotherapy at other timepoints (p=0.86). In multivariable analysis, delay in receipt of adjuvant chemotherapy beyond 45 days was not associated with inferior survival (Table 2). When time to adjuvant therapy was modeled as a continuous variable, increasing time was not associated with a difference in survival (Table 3). Among patients with non-squamous pathology and lymph-node positive disease (n=1187), the lack of association between timing and survival was preserved (Table 4).
Figure 3.
Overall survival for patients with esophageal cancer, stratified by time to adjuvant chemotherapy following esophagectomy (Q1 ≤45 days, Q2 46–60 days, Q3 61–75 days, Q4 >75 days). Q=quartile.
Table 2.
Cox multivariable regression of independent predictors of survival, with time to adjuvant therapy modeled as a categorical variable by quartile.
| Predictor | Hazard Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age (per year) | 1.01 | 1.00 | 1.02 | 0.03 |
| Sex (female) | 0.93 | 0.76 | 1.13 | 0.45 |
| Race (reference: White) | ||||
| Black | 1.15 | 0.83 | 1.59 | 0.41 |
| Other | 1.01 | 0.63 | 1.62 | 0.96 |
| Year of diagnosis (per year) | 0.94 | 0.92 | 0.96 | <0.001 |
| Charlson-Deyo Comorbidity (CDCC) score (reference: 0) | ||||
| 1 | 1.08 | 0.92 | 1.27 | 0.34 |
| 2+ | 1.40 | 1.06 | 1.85 | 0.02 |
| Insurance status (reference: private) | ||||
| Government | 1.20 | 1.01 | 1.42 | 0.03 |
| None | 1.19 | 0.70 | 2.02 | 0.52 |
| Facility location (reference: metro) | ||||
| Urban | 1.28 | 1.07 | 1.54 | 0.007 |
| Rural | 1.12 | 0.73 | 1.73 | 0.60 |
| Facility type (reference: non-academic) | ||||
| Academic/Research Program | 0.97 | 0.84 | 1.11 | 0.63 |
| Pathologic stage (reference: 1) | ||||
| 2 | 2.36 | 1.25 | 4.48 | 0.008 |
| 3 | 4.04 | 2.14 | 7.62 | <0.001 |
| Margin status (reference: R0) | ||||
| R1 | 1.65 | 1.37 | 1.99 | <0.001 |
| R2 | 1.29 | 0.53 | 3.16 | 0.58 |
| Squamous histology | 0.99 | 0.80 | 1.23 | 0.94 |
| Perioperative radiation (reference: none) | ||||
| Induction | 0.49 | 0.83 | 1.13 | 0.17 |
| Adjuvant | 0.97 | 0.83 | 1.13 | 0.68 |
| Length of stay | 1.00 | 0.99 | 1.01 | 0.77 |
| 30-day readmission | 1.27 | 0.97 | 1.65 | 0.08 |
| Time to adjuvant therapy (reference: Q1 ≤45 days) | ||||
| Q2 46–60 days | 1.08 | 0.90 | 1.31 | 0.40 |
| Q3 61–75 days | 1.17 | 0.96 | 1.43 | 0.12 |
| Q4 >75 days | 1.07 | 0.89 | 1.30 | 0.46 |
Table 3.
Cox multivariable regression of independent predictors of survival, with time to adjuvant therapy modeled as a continuous variable.
| Predictor | Hazard Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age (per year) | 1.01 | 1.00 | 1.02 | 0.03 |
| Sex (female) | 0.93 | 0.76 | 1.13 | 0.44 |
| Race (reference: White) | ||||
| Black | 1.14 | 0.82 | 1.57 | 0.45 |
| Other | 1.03 | 0.65 | 1.65 | 0.89 |
| Year of diagnosis (per year) | 0.94 | 0.92 | 0.97 | <0.001 |
| Charlson-Deyo Comorbidity (CDCC) score (reference: 0) | ||||
| 1 | 1.08 | 0.92 | 1.27 | 0.33 |
| 2+ | 1.41 | 1.07 | 1.87 | 0.02 |
| Insurance status (reference: private) | ||||
| Government | 1.20 | 1.02 | 1.42 | 0.03 |
| None | 1.19 | 0.71 | 2.02 | 0.51 |
| Facility location (reference: metro) | ||||
| Urban | 1.29 | 1.08 | 1.55 | 0.005 |
| Rural | 1.11 | 0.72 | 1.71 | 0.63 |
| Facility type (reference: non-academic) | ||||
| Academic/Research Program | 0.98 | 0.85 | 1.12 | 0.72 |
| Pathologic stage (reference: 1) | ||||
| 2 | 2.38 | 1.25 | 4.50 | 0.008 |
| 3 | 4.04 | 2.14 | 7.64 | <0.001 |
| Margin status (reference: R0) | ||||
| R1 | 1.64 | 1.36 | 1.98 | <0.001 |
| R2 | 1.28 | 0.53 | 3.12 | 0.58 |
| Squamous histology | 1.00 | 0.80 | 1.24 | 0.98 |
| Perioperative radiation (reference: none) | ||||
| Induction | 0.48 | 0.18 | 1.31 | 0.15 |
| Adjuvant | 0.97 | 0.83 | 1.13 | 0.71 |
| Length of stay | 1.00 | 0.99 | 1.01 | 0.79 |
| 30-day readmission | 1.26 | 0.97 | 1.64 | 0.08 |
| Time to adjuvant therapy (per day increase) | 1.00 | 1.00 | 1.00 | 0.83 |
Table 4.
Cox Multivariable regression of subgroup analysis in 1187 patients with non-squamous and pathologically lymph node-positive disease. The results below represent the output from two separate multivariable Cox models, one treating time to adjuvant therapy as a categorical variable and the other as a continuous variable using the same covariates as in Tables 2 and 3 with the exclusion of the histology variable.
| Predictor | Hazard Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Time to adjuvant therapy (reference: Q1 ≤45 days) | ||||
| Q2 46–60 days | 1.10 | 0.88 | 1.36 | 0.40 |
| Q3 61–75 days | 1.17 | 0.93 | 1.47 | 0.19 |
| Q4 >75 days | 1.03 | 0.83 | 1.29 | 0.78 |
| Time to adjuvant therapy (per day increase) | 0.9996 | 0.9966 | 1.0026 | 0.80 |
Comment
In this analysis of the NCDB, we found that the timing of adjuvant chemotherapy following esophagectomy did not impact survival, even after adjusting for pathologic stage and margin status among patients who did not receive any neoadjuvant chemotherapy. Our study suggests that delay in adjuvant therapy beyond two months following surgery for esophageal cancer does not worsen survival. We advocate that adjuvant therapy be considered when appropriate, but that initiation can be delayed to accommodate an individual patient’s continued recovery from surgery when necessary.
The utility of adjuvant therapy for esophageal cancer has been previously established. The INT-0116 trial demonstrated that adjuvant therapy in combination with surgery, significantly prolongs survival and reduces recurrence for esophagogastric adenocarcinoma (17, 18). These results have been supported by retrospective studies and meta-analysis (19). It is important to note that our study did not compare survival for receipt of adjuvant therapy at different timepoints to those who did not receive it at all. Rather, we address the paucity of literature regarding the timing of adjuvant therapy in this population.
To our knowledge, this is the first analysis examining the effect of timing of adjuvant chemotherapy on survival following esophagectomy. There are many retrospective cohort analyses examining the impact of timing of surgery following neoadjuvant therapy in esophageal cancer, with conflicting results (11–14). In the management of other malignancies, the timing between surgery and adjuvant therapy has been fiercely debated. This interval is well studied in stage III colon cancer, where the optimal initiation of adjuvant chemotherapy is 6–8 weeks following surgery (4, 6, 10). Similar studies in breast cancer advocate for timely adjuvant therapy, demonstrating that delays compromise survival (8). Alternatively, timing of adjuvant therapy does not appear to impact survival in patients with non-small cell lung cancer (7, 9). Our study expands this area of work and demonstrates similar findings in esophageal cancer.
In the management of esophageal cancer, the timing of adjuvant chemotherapy following esophagectomy is a complex decision predicated on postoperative recovery and fitness for adjuvant therapy, especially given the morbidity associated with esophagectomy (20–22). At all times during the treatment course, multidisciplinary decision making must consider patient performance status and quality of life, as they are associated with survival in patients with esophageal cancer (23–25). Both patient-perceived quality of life and physician-measured performance status (such as ECOG) have been studied as prognostic indicators for patients undergoing chemotherapy in the adjuvant and palliative settings. In esophageal cancer, Blazeby et al. described that patients with increased physical functioning had decreased likelihood of death at any given time during treatment (23). Additional studies have described fatigue, reflux, and appetite as prognostic quality of life measures in esophageal cancer patients (24, 25). Given that patients encounter many of these symptoms, as well as reduced physical functioning, in the immediate post-esophagectomy period, delaying adjuvant therapy until patients are stronger may be the wise clinical decision. Further, chemoradiation is physically taxing and has many associated toxicities. From this analysis, we propose that the timing of adjuvant therapy is not independently associated with survival, and that patients should be optimized prior to receipt of adjuvant therapy following esophagectomy.
Our study has several limitations. As a retrospective cohort analysis of a large registry, the most important limitation is selection bias for which we could not entirely adjust for. For instance, we do not know the reasons driving the timing of adjuvant therapy in our cohort. It is likely that patients who underwent uneventful recovery received adjuvant therapy early compared to those with postoperative complications – of which we are unable to account for despite attempts to adjust for this by inclusion of postoperative length of stay and unexpected 30-day readmission in our regression models. Additionally, while many patients receive induction therapy prior to esophagectomy (CROSS trial), guideline-concordant care is not universal and our study population likely represents a mixture of patients receiving guideline-concordant and discordant care. Unfortunately, we are not privy to the reasons that some patients receive guideline-discordant care and this may further impact selection bias. We were also limited by the variables available in the NCDB. For instance, the NCDB does not catalogue postoperative performance status, nutrition, complications, and information about chemotherapeutic regimens or doses. Additionally, we were unable to evaluate recurrence, recurrence-free survival, and disease-specific survival. Despite these limitations, our study provides a national perspective with large sample size on a previously unstudied clinical dilemma.
In this NCDB analysis, we found that the timing of adjuvant therapy does not have an impact on survival following esophagectomy among patients who did not receive any neoadjuvant therapy. Delay in the initiation of adjuvant chemotherapy beyond even 75 days is not associated with inferior survival. Given the highly variable post-operative course following esophagectomy, the decision to start adjuvant chemotherapy should involve multidisciplinary discussion and be made on a patient-by-patient basis. Thoracic surgeons should reinforce the importance of recovery and advocate for return to baseline performance status prior to their patients pursuing adjuvant chemotherapy.
Supplementary Material
Acknowledgements and Funding
The American College of Surgeons is in a Business Associate Agreement that includes a data use agreement with each of its Commission on Cancer accredited hospitals. The data used in the study are derived from a de-identified National Cancer Data Base file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used or the conclusions drawn from these data by the investigators.
Dr. Raman was supported by a National Institutes of Health T-32 grant 5T32CA093245 in surgical oncology. Dr. Jawitz was supported by a National Institutes of Health T-32 grant 5T32HL069749 in clinical research.
Footnotes
Disclosures
BCT is a consultant for Medtronic, Inc.
References
- 1.Birkmeyer JD, Siewers AE, Finlayson EVA et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346(15):1128–1137. [DOI] [PubMed] [Google Scholar]
- 2.Luketich JD, Pennathur A, Franchetti Y et al. Minimally invasive esophagectomy: Results of a prospective phase II multicenter trial-the eastern cooperative oncology group (E2202) study. Ann Surg 2015;261(4):702–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network (NCCN). 2018 Esophageal and esophagogastric junction cancers (version 2.2018). Available at https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf Accessed 2019 February 15. [DOI] [PubMed]
- 4.Turner MC, Farrow NE, Rhodin KE et al. Delay in adjuvant chemotherapy and survival advantage in stage III colon cancer. J Am Coll Surg 2018;226(4):670–678. [DOI] [PubMed] [Google Scholar]
- 5.Aloia TA, Zimmitti G, Conrad C, Gottumukalla V, Kopetz S, Vauthey JN. Return to intended oncologic treatment (RIOT): A novel metric for evaluating the quality of oncosurgical therapy for malignancy. J Surg Oncol 2014;110(2):107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: A systematic review and meta-analysis. JAMA 2011;305(22):2335–2342. [DOI] [PubMed] [Google Scholar]
- 7.Booth CM, Shepherd FA, Peng Y et al. Time to adjuvant chemotherapy and survival in non-small cell lung cancer: A population-based study. Cancer 2013;119(6):1243–1250. [DOI] [PubMed] [Google Scholar]
- 8.Raphael MJ, Biagi JJ, Kong W, Mates M, Booth CM, Mackillop WJ. The relationship between time to initiation of adjuvant chemotherapy and survival in breast cancer: A systematic review and meta-analysis. Breast Cancer Res Treat 2016;160(1):17–28. [DOI] [PubMed] [Google Scholar]
- 9.Salazar MC, Rosen JE, Wang Z et al. Association of delayed adjuvant chemotherapy with survival after lung cancer surgery. JAMA Oncol 2017;3(5):610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Des Guetz G, Nicolas P, Perret GY, Morere JF, Uzzan B. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer 2010;46(6):1049–1055. [DOI] [PubMed] [Google Scholar]
- 11.Qin Q, Xu H, Liu J et al. Does timing of esophagectomy following neoadjuvant chemoradiation affect outcomes? A meta-analysis. Int J Surg 2018;59:11–18. [DOI] [PubMed] [Google Scholar]
- 12.Franko J, Voynov G, Goldman CD. Esophagectomy timing after neoadjuvant therapy for distal esophageal adenocarcinoma. Ann Thorac Surg 2016;101(3):1123–1130. [DOI] [PubMed] [Google Scholar]
- 13.Tsang JS, Tong DKH, Lam KO et al. Appropriate timing for surgery after neoadjuvant chemoradiation for esophageal cancer. Dis Esophagus 2017;30(9):1–8. [DOI] [PubMed] [Google Scholar]
- 14.Ranney DN, Mulvihill MS, Yerokun BA et al. Surgical resection after neoadjuvant chemoradiation for oesophageal adenocarcinoma: What is the optimal timing? Eur J Cardiothorac Surg 2017;52(3):543–551. [DOI] [PubMed] [Google Scholar]
- 15.Raval MV, Bilimoria KY, Stewart AK, Bentrem DJ, Ko CY. Using the NCDB for cancer care improvement: An introduction to available quality assessment tools. J Surg Oncol 2009;99(8):488–490. [DOI] [PubMed] [Google Scholar]
- 16.Winchester DP, Stewart AK, Phillips JL, Ward EE. The national cancer data base: Past, present, and future. Ann Surg Oncol 2010;17(1):4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macdonald JS, Smalley SR, Benedetti J et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345(10):725–730. [DOI] [PubMed] [Google Scholar]
- 18.Smalley SR, Benedetti J, Haller DG et al. Updated analysis of swog-directed intergroup study 0116: A phase iii trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol 2012;30(19):2327–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang J, Chang JY, Sun X, Men Y, Zeng H, Hui Z. Role of postoperative concurrent chemoradiotherapy for esophageal carcinoma: A meta-analysis of 2165 patients. J Cancer 2018;9(3):584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raymond DP, Seder CW, Wright CD et al. Predictors of major morbidity or mortality after resection for esophageal cancer: A society of thoracic surgeons general thoracic surgery database risk adjustment model. Ann Thorac Surg 2016;102(1):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kassis ES, Kosinski AS, Ross P, Koppes KE, Donahue JM, Daniel VC. Predictors of anastomotic leak after esophagectomy: An analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg 2013;96(6):1919–1926. [DOI] [PubMed] [Google Scholar]
- 22.Grimminger PP, Goense L, Gockel I et al. Diagnosis, assessment, and management of surgical complications following esophagectomy. Ann NY Acad Sci 2018;1434(1):254–273. [DOI] [PubMed] [Google Scholar]
- 23.Blazeby JM, Brookes ST, Alderson D. The prognostic value of quality of life scores during treatment for oesophageal cancer. Gut 2001;49(2):227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergquist H, Johnsson A, Hammerlid E, Wenger U, Lundell L, Ruth M. Factors predicting survival in patients with advanced oesophageal cancer: A prospective multicentre evaluation. Aliment Pharmacol Ther 2008;27(5):385–395. [DOI] [PubMed] [Google Scholar]
- 25.McKernan M, McMillan DC, Anderson JR, Angerson WJ, Stuart RC. The relationship between quality of life (eortc qlq-c30) and survival in patients with gastro-oesophageal cancer. Br J Cancer 2008;98(5):888–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.