Abstract
Significance.
This study shows that non-visual mechanism(s) can guide chick eyes to recover from myopia or hyperopia bi-directionally to regain their age-matched length. Since eye growth control is phylogenetically conserved across many species, it is possible that in general, emmetropization mechanisms are not exclusively based on a local visual feedback system.
Purpose.
Across species, growing eyes compensate for imposed defocus by modifying their growth, showing the visual controls on eye growth and emmetropization. When the spectacle lens is removed, the eyes rapidly recover back to a normal size similar to that in the untreated eyes. We asked whether this recovery process was dependent on visual feedback, or whether it might be guided by intrinsic non-visual mechanisms.
Methods.
Chicks wore either a +7 D (n=16) or −7 D (n=16) lens over one eye for 4-7 days; the fellow eye left untreated. After lens removal, half were recovered in darkness and half in white light. Refractive error and ocular dimensions were measured before and after lens treatment, and after recovery with a Hartinger refractometer and A-scan biometer, respectively.
Results.
While chick eyes completely recovered from prior lens treatment under normal light after 2 days, they also partially recovered from prior hyperopia (by 60%) and myopia (by 69%) after being kept in darkness for 3 days: A +7D and −7D lens induced a difference between the eyes of +7.08D and −4.69D, respectively. After recovery in darkness, the eyes recovered by 3.18D and 2.88D, respectively.
Conclusions.
In the absence of visual cues, anisometropic eyes can modify and reverse their growth to regain a similar length to their fellow untreated eye. Since eye growth control is phylogenetically conserved across many species, it is possible that non-visual mechanisms may contribute more generally to emmetropization, and that recovery from anisometropic refractive errors may not be wholly visually controlled.
Numerous animal studies over the last four decades have established that post-natal growth is actively controlled by visual signals.1 To clearly focus distant objects on the retina, the physical length of the eye must match its optical length (emmetropia). In multiple species, eyes emmetropize and compensate for imposed defocus by changing their axial growth during development2, as demonstrated in fish,3, 4 chicks,5, 6 rhesus macaques,7 marmosets,8 tree shrews,9 guinea pigs,10 and particular genetic strains of mice (eg: Balb/cJ).11 Additionally, bidirectional choroidal changes accompany compensation to defocus (chicks,12, 13 tree shrews,14 marmosets,15 rhesus macaques,16 guinea pigs,17, 18 humans.19-22). In chicks the choroidal changes are so large that it also effectively changes the location of the retinal plane13, while for other species it is generally thought to implicate a ubiquitous mechanism that is an important factor in the cascade from visual stimulation to scleral changes which affect eye size. It has also been show that eyes can recover from prior lens treatment after lens removal in tree shrews,23 rhesus macaques,7,24,25 chicks,5,6,13 marmosets,26,27 guinea pigs,28 and mice.29,30
Both lens compensation and recovery from lens compensation strongly support the notion that the eye can detect both the magnitude and the sign of defocus and use this information to guide its growth.2Furthermore, since in chicks, a brief episode of myopic and hyperopic defocus can induce rapid choroidal compensation that does not initially change the eye’s refractive error, it has been suggested that chick eyes can distinguish between myopic and hyperopic defocus from the beginning of lens treatment, instead of using a trial-and-error mechanism.31 While it is still unclear exactly which aspects of the visual information or what cues the eye uses to guide its growth, longitudinal chromatic aberration,32 higher-order chromatic aberrations33 and astigmatism,34 among others, have been suggested.
Several studies have suggested that non-visual factors may be involved. First, even though prolonged dark rearing causes chick eyes to become hyperopic due to corneal flattening they also elongate far more than normal partially offsetting the hyperopia despite the lack of visual input.35 Secondly, chick eyes recovered from form deprivation myopia even when the refractive error was optically corrected with negative lenses of the appropriate power.36 Furthermore, guinea pigs can partially recover from form deprivation myopia after 3 days of darkness (by 35%) primarily by inhibiting their axial elongation (by 26%).37 Most dramatically, chick eyes made myopic in only the nasal retina (thus very asymmetric in shape) recovered normal symmetry even after the retina has been lesioned by tunicamycin, a powerful inhibitor of protein glycosylation that causes apoptotic cell death in retinal ganglion cells.38
It is hypothesized in the current study that in addition to visual input (defocus), there are non-visual mechanisms or factors that act to guide an anisometropic eye to return to its normal, age-appropriate eye size or shape in the absence of any visual input. To investigate the existence of such non-visual factors, the current study determined whether vision is necessary for the recovery process after lens compensation by observing recovery from prior positive or negative lens treatment while chicks were kept in darkness compared to those recovered in the light.Some of these results have been presented in an abstract form (Zhu X, Wallman J, and McFadden SA. IOVS 2016;57:ARVO E-Abstract 3791).
METHODS
Animals
White leghorn chicks (n = 32) were hatched from eggs obtained from Cornell University (Cornell K-strain; Ithaca, NY). Once hatched, all chicks were housed in heated brooders (91 x 76 cm), with a 14:10 hour light:dark cycle under daylight balanced fluorescent illumination (approximately 300 lux, lights off from 10 pm to 8 am) prior to the start of experiments, with food and water ad libitum.39 Chicks were kept in heated, sound-attenuated chambers (76 x 61 cm) during experiments, with a 14:10 hour light:dark cycle under daylight balanced fluorescent illumination, unless otherwise specified. Care and use of all animals adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. The protocols used were approved by the Institutional Animal Care and Use Committee at the City College of the City University of New York.
Experimental Procedures
One-to-7-day old chicks (based on availability) were raised in the light with a lens worn in front of one eye, and then lenses were removed, and animals were kept in either light or dark conditions. In four separate groups, chicks wore either a +7 D (the +7-light and +7-dark groups, n = 8 for each group) or a −7 D (the −7-light group, n = 5; the −7-dark group, n = 11) spectacle lens in front of one eye (the experimental eye, X) for at least 4 days (sufficient to induce robust compensation), and the other eye was left as the untreated control (the fellow eye, N).
Lenses were glass with a diameter of 12 mm as previously described.39 Each lens was glued onto a rigid plastic ring backed with Velcro. The lens was then attached to a matching Velcro ring glued to the feathers around the eyes.39 This allowed the lenses to be quickly replaced with clean lenses each day while the animals were very briefly kept in darkness.
To induce recovery, lenses were removed at various ages (see Table 1), and animals either continued to be held in their normal 14:10 hour light:dark cycle for 2 days to allow visual input to guide recovery (the +7-light and −7-light groups), or were put into complete darkness in a light-proof chamber for 3 days (the +7-dark and −7-dark groups) to eliminate any visually-based recovery mechanisms. During dark rearing, chicks’ crops were checked daily to make sure that they were eating normally, and no issues were discovered.
Table 1.
Treatment details
| Group | Details (age in days) | Physiological age (Days) |
Normalized period (Days) |
n |
|---|---|---|---|---|
| +7-light | +7 D lens wear for 7 days in light | 7 to 14 | −7 to 0 | 8 |
| Removal of lens for 2 days in light | 14 to 16 | 0 to 2 | ||
| +7-dark | +7 D lens wear for 4 days in light | 7 to 11 | −4 to 0 | 8 |
| Removal of lens for 3 days in darkness | 11 to 14 | 0 to 3 | ||
| −7-light | −7 D lens wear for 5 days in light | 1 to 6 | −5 to 0 | 5 |
| Removal of lens for 2 days in light | 6 to 8 | 0 to 2 | ||
| −7-dark | −7 D lens wear for 4 days in light | 7 to 11 | −4 to 0 | 11 |
| Removal of lens for 3 days in darkness | 11 to 14 | 0 to 3 |
n = sample size; D = diopter
Refractions and biometry measurements were taken before and after lens treatment, and again after a recovery period, all under dim lighting conditions (Table 1).
To account for the slightly different ages of animals, times were normalized to the start of lens removal (see Table 1 for details). These normalized times are referred to in the results text rather than the physiological ages.
Measurements
Measures were made in both the experimental and fellow untreated eyes. The Spherical Equivalent Refractive Error in diopters (D) was calculated as half of the cylindrical power added to the spherical power, as measured during anesthesia with a Hartinger refractometer (Jena Coincidence Refractometer, Carl Zeiss, Jena, Germany) modified for small pupils, as previously described.40 Chicks were anaesthetized with 1.5% of isoflurane in oxygen41 without cycloplegic agents, prior to the refraction measurement.
Internal ocular dimensions on-axis were measured during anesthesia using A-scan ultrasonography. A-scan was conducted with a 30 MHz transducer (Panametrics Model 176599) and sampled at 100 MHz with a Sonix 8100 A/D board.42 The internal ocular dimensions consisted of the anterior chamber depth, lens thickness, vitreous chamber depth, retinal thickness, choroidal thickness, and scleral thickness. Axial length was defined as the sum of anterior chamber depth, lens thickness, vitreous chamber depth, and the thicknesses of the retina, choroid, and sclera.
Analyses
Data are shown as the mean ± SEM for the experimental (X) and fellow (N) eyes and the mean ± SEM of the interocular differences between the two eyes (X – N) in Table 2. The growth (or shrinkage) in various ocular parameters was also calculated for each eye (ΔX and ΔN) during the lens-wear and recovery periods and the difference between the two eyes in their changes is referred to as the relative change (ΔX - ΔN). These differences are commonly used to correct for natural variations in the size of fellow eyes between individual animals.
Table 2.
Summary of ocular dimensions and refractive error (X and N) and inter-ocular difference (X – N) on various days (Mean ± SEM).
| Group | Time period relative to lens removal (day)* |
Eye | Anterior chamber depth (mm) | Lens thickness (mm) | Vitreous chamber depth (mm) | Choroidal thickness (mm) | Axial length (mm) | Refractive error (D) |
|---|---|---|---|---|---|---|---|---|
|
+7 D recovery in light (+7-light) n = 8 |
−7 (baseline) | X | 1.20 ± 0.01 | 1.85 ± 0.0 | 5.15 ± 0.08 | 0.19 ± 0.02 | 8.72 ± 0.09 | −0.69 ± 0.33 |
| N | 1.22 ± 0.00 | 1.85 ± 0.03 | 5.15 ± 0.07 | 0.16 ± 0.01 | 8.69 ± 0.08 | −0.23 ± 0.45 | ||
| X – N | −0.02 ± 0.01 | 0.00 ± 0.01 | 0.00 ± 0.01 | 0.03 ± 0.03 | 0.03 ± 0.03 | −0.46 ± 0.49 | ||
| p | .072 | .782 | .855 | .086 | .393 | .393 | ||
| 0 (lens removal) | X | 1.37 ± 0.02 | 2.16 ± 0.02 | 5.02 ± 0.08 | 0.28 ± 0.02 | 9.18 ± 0.10 | 6.57 ± 0.41 | |
| N | 1.36 ± 0.02 | 2.17 ± 0.02 | 5.31 ± 0.07 | 0.20 ± 0.01 | 9.37 ± 0.07 | 0.23 ± 0.45 | ||
| X – N | 0.00 ± 0.01 | −0.01 ± 0.01 | −0.29 ± 0.03 | 0.09 ± 0.02 | −0.19 ± 0.03 | 6.34 ± 0.71 | ||
| p | .731 | .445 | <.001 | <.001 | <.001 | <.001 | ||
| 2 (recovery) | X | 1.42 ± 0.01 | 2.24 ± 0.02 | 5.38 ± 0.05 | 0.21 ± 0.01 | 9.59 ± 0.07 | 0.52 ± 0.41 | |
| N | 1.42 ± 0.01 | 2.30 ± 0.01 | 5.43 ± 0.05 | 0.20 ± 0.00 | 9.68 ± 0.06 | 0.25 ± 0.36 | ||
| X – N | 0.00 ± 0.01 | −0.05 ± 0.01 | −0.05 ± 0.02 | 0.01 ± 0.01 | −0.09 ± 0.03 | 0.27 ± 0.49 | ||
| p | .829 | <.001 | .02 | .741 | .003 | .614 | ||
|
+7 D recovery in darkness (+7-dark) n = 8 |
−4 (baseline) | X | 1.28 ± 0.02 | 1.88 ± 0.02 | 5.05 ± 0.03 | 0.20 ± 0.02 | 8.74 ± 0.05 | −0.52 ± 0.69 |
| N | 1.30 ± 0.02 | 1.87 ± 0.01 | 5.05 ± 0.04 | 0.18 ± 0.02 | 8.73 ± 0.04 | −1.41 ± 0.32 | ||
| X – N | −0.02 ± 0.01 | 0.01 ± 0.01 | 0.00 ± 0.01 | 0.02 ± 0.02 | 0.01 ± 0.02 | 0.89 ± 0.65 | ||
| p | .386 | .581 | .920 | .186 | .760 | .348 | ||
| 0 (lens removal) | X | 1.25 ± 0.03 | 1.94 ± 0.02 | 4.91 ± 0.04 | 0.29 ± 0.01 | 8.72 ± 0.07 | 5.75 ± 0.85 | |
| N | 1.32 ± 0.02 | 1.98 ± 0.02 | 5.14 ± 0.05 | 0.16 ± 0.01 | 8.92 ± 0.06 | −1.33 ± 0.33 | ||
| X – N | −0.06 ± 0.02 | −0.04 ± 0.01 | −0.23 ± 0.02 | 0.12 ± 0.02 | −0.20 ± 0.03 | 7.08 ± 0.80 | ||
| p | .005 | .041 | <.001 | <.001 | <.001 | <.001 | ||
| 3 (recovery) | X | 1.37 ± 0.04 | 2.08 ± 0.02 | 5.21 ± 0.04 | 0.18 ± 0.00 | 9.17 ± 0.08 | 1.22 ± 1.02 | |
| N | 1.43 ± 0.02 | 2.10 ± 0.02 | 5.29 ± 0.06 | 0.13 ± 0.00 | 9.29 ± 0.06 | −2.68 ± 0.53 | ||
| X – N | −0.07 ± 0.03 | −0.02 ± 0.02 | −0.08 ± 0.04 | 0.05 ± 0.00 | −0.12 ± 0.04 | 3.90 ± 0.75 | ||
| p | .004 | .119 | <.001 | .003 | <.001 | <.001 | ||
|
−7 D recovery in light (−7-light) n = 5 |
−5 (baseline) | X | 1.22 ± 0.03 | 1.60 ± 0.01 | 5.18 ± 0.10 | 0.23 ± 0.03 | 8.55 ± 0.11 | −2.06 ± 0.89 |
| N | 1.22 ± 0.03 | 1.60 ± 0.01 | 5.10 ± 0.07 | 0.25 ± 0.03 | 8.49 ± 0.08 | −2.20 ± 1.01 | ||
| X – N | 0.00 ± 0.01 | 0.00 ± 0.00 | 0.08 ± 0.04 | −0.02 ± 0.02 | 0.06 ± 0.04 | 0.14 ± 0.41 | ||
| p | .94 | .985 | .024 | .337 | .199 | .718 | ||
| 0 (lens removal) | X | 1.20 ± 0.04 | 1.86 ± 0.02 | 5.43 ± 0.06 | 0.14 ± 0.01 | 8.95 ± 0.10 | −5.78 ± 0.79 | |
| N | 1.24 ± 0.02 | 1.91 ± 0.02 | 5.01 ± 0.05 | 0.20 ± 0.01 | 8.69 ± 0.06 | −1.16 ± 0.47 | ||
| X – N | −0.05 ± 0.03 | −0.05 ± 0.02 | 0.42 ± 0.02 | −0.06 ± 0.01 | 0.25 ± 0.05 | −4.62 ± 0.71 | ||
| p | .151 | .904 | <.001 | .029 | <.001 | <.001 | ||
| 2 (recovery) | X | 1.27 ± 0.05 | 1.94 ± 0.02 | 5.20 ± 0.07 | 0.35 ± 0.02 | 9.08 ± 0.12 | −0.06 ± 0.28 | |
| N | 1.31 ± 0.02 | 1.96 ± 0.01 | 5.04 ± 0.06 | 0.23 ± 0.04 | 8.87 ± 0.09 | −1.37 ± 0.48 | ||
| X – N | −0.04 ± 0.04 | −0.02 ± 0.02 | 0.16 ± 0.03 | 0.12 ± 0.02 | 0.21 ± 0.05 | 1.31 ± 0.33 | ||
| p | .058 | .017 | <.001 | .017 | <.001 | <.001 | ||
|
−7 D recovery in darkness (−7-dark) n = 11 |
−4 (baseline) | X | 1.32 ± 0.02 | 1.92 ± 0.02 | 5.01 ± 0.06 | 0.22 ± 0.03 | 8.82 ± 0.04 | −0.69 ± 0.42 |
| N | 1.31 ± 0.01 | 1.93 ± 0.02 | 5.03 ± 0.05 | 0.21 ± 0.02 | 8.82 ± 0.04 | −0.19 ± 0.23 | ||
| X – N | 0.01 ± 0.01 | −0.01 ± 0.01 | −0.02 ± 0.02 | 0.01 ± 0.02 | −0.01 ± 0.02 | −0.50 ± 0.32 | ||
| p | .677 | .526 | .293 | .814 | .858 | .393 | ||
| 0 (lens removal) | X | 1.34 ± 0.02 | 2.02 ± 0.02 | 5.28 ± 0.07 | 0.17 ± 0.02 | 9.17 ± 0.07 | −5.22 ± 0.46 | |
| N | 1.35 ± 0.01 | 2.04 ± 0.02 | 5.09 ± 0.07 | 0.23± 0.03 | 9.05 ± 0.06 | −0.52 ± 0.33 | ||
| X – N | −0.01 ± 0.02 | −0.02 ± 0.01 | 0.20 ± 0.02 | −0.05 ± 0.03 | 0.11 ± 0.03 | −4.69 ± 0.47 | ||
| p | .835 | .184 | <.001 | .027 | <.001 | <.001 | ||
| 3 (recovery) | X | 1.40 ± 0.03 | 2.13 ± 0.02 | 5.36 ± 0.07 | 0.18 ± 0.00 | 9.42 ± 0.07 | −2.92 ± 0.53 | |
| N | 1.46 ± 0.02 | 2.17 ±0.02 | 5.23 ± 0.06 | 0.19 ± 0.02 | 9.40 ± 0.05 | −1.11 ± 0.46 | ||
| X – N | −0.05 ± 0.04 | −0.03 ± 0.02 | 0.14 ± 0.02 | −0.01 ± 0.02 | 0.02 ± 0.04 | −1.81 ± 0.56 | ||
| p | .036 | .069 | <.001 | .715 | .523 | .002 |
Normalized age used. See Table 1 and Methods for details. D = diopter.
P: The mean values in the experimental and fellow eyes were compared at different time points measured using Two-Way Mixed Measures Analysis of Variance (ANOVA), with the Holm-Sidak adjustment method. P-values of statistical significance are underlined and in bold font.
One-Way Repeated Measures ANOVA (SigmaPlot V12.5) with post hoc comparisons (Holm-Sidak adjustment) were used to compare the mean interocular differences (X – N) at different time points within the same group. Two-Way Mixed Measures ANOVA (together with Holm Sidak comparisons) were used to compare mean values between the experimental and fellow eyes on various days (X vs. N) at different time points.
Two-tailed, Student’s t-tests were used to compare the change in ocular parameters (ΔX vs. ΔN). Percentage of recovery was defined as the ratio between the relative change during lens compensation and that during recovery.
RESULTS
In summary, without any visual cues, chick eyes significantly recovered from prior lens treatment while kept in darkness. While chick eyes rapidly recovered from prior hyperopia or myopia after only 2 days in normal light, they only partially recovered when kept in darkness for 3 days. Values for each eye and the differences between the two eyes (X – N) are shown in Table 2.
Recovery from Prior Positive Lens Wear
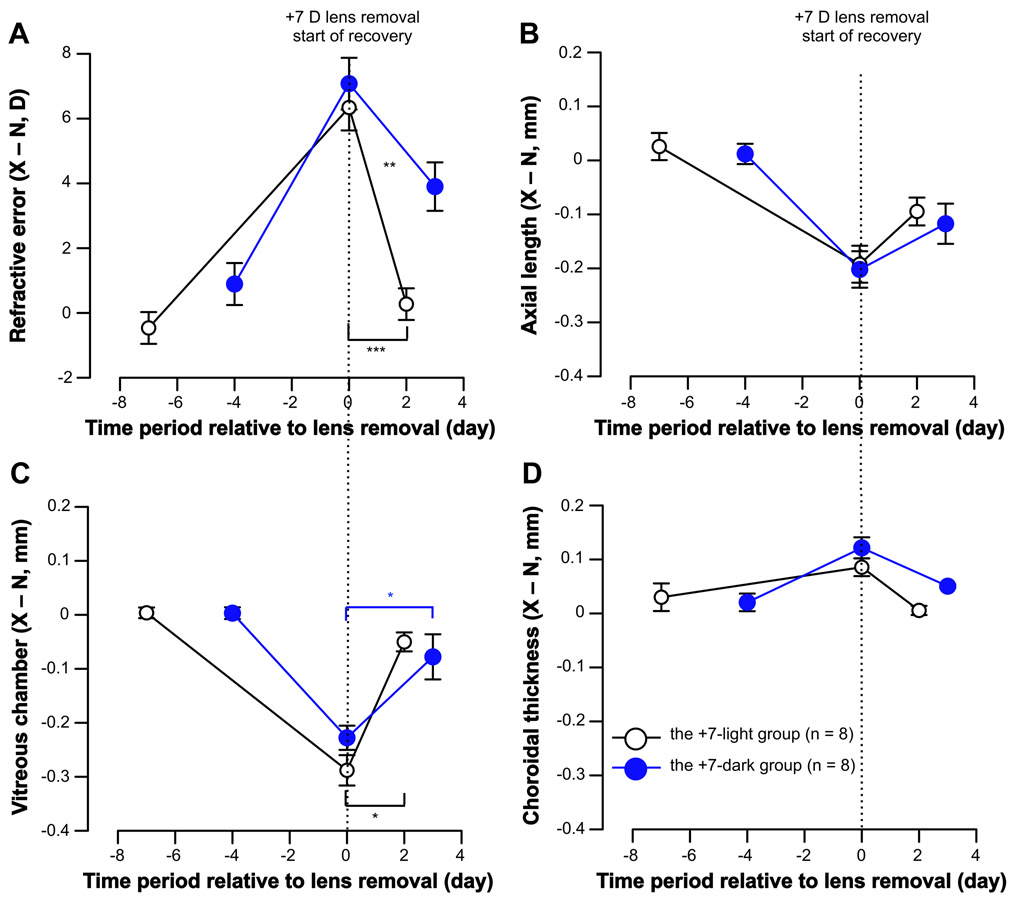
Wearing a +7 D lens over one eye for either 7 (the +7-light group) or 4 days (the +7-dark group) caused robust compensation, and the lens-wearing eyes became significantly more hyperopic than the fellow eye: The mean difference between the two eyes at the end of the lens-wear period (X – N at day 0) was +6.34 (± 0.71) D and +7.08 (± 0.80) D respectively for these two groups (P < .001 for both, Fig. 1A). This robust hyperopic shift was caused by a relatively smaller axial length in the lens-wearing eye (the +7-light group: −0.19 ± 0.03 mm, the +7-dark group: −0.20 ± 0.03 mm; P < .001 for both, Fig. 1B) and shorter vitreous chamber depth (the +7-light group: −0.29 ± 0.03 mm, the +7-dark group: −0.23 ± 0.02 mm; P < .001 for both, Fig. 1C), and thicker choroids (the +7-light group: +0.09 ± 0.02 mm, the +7-dark group: +0.12 ± 0.02 mm; P < .001 for both, Fig. 1D). These inter-ocular differences at the end of lens-wear (day 0 in Fig. 1) were similar in these two groups (refractive error: P = .503, axial length: P = .842, vitreous chamber depth: P = .107, choroidal thickness: P = .138, Holm-Sidak comparisons after ANOVA).
Figure 1.
Comparison of recovery in the light and the dark after positive lens wear. Data is shown as the inter-ocular difference between the experimental and fellow eyes (X – N, Mean ± SEM) at various ages for (A) refractive error, (B) axial length, (C) vitreous chamber depth, and (D) choroidal thickness. Age has been normalized so the day of lens removal (the start of recovery) is represented by zero on the X-axes. Asterisks with bars indicate statistically significant difference for inter-ocular difference (X – N) between various ages in each group (* p < .05, *** p < .001, One-Way Repeated Measures ANOVA), and asterisks without bars indicate statistically significant difference for relative difference (ΔX – ΔN) between the +7-light and +7-dark groups during recovery (** p < .01, unpaired, 2-tailed Student’s t-test).
Two days after the positive lenses were removed, chick eyes that were exposed to normal light (the +7-light group) recovered by 89% from prior hyperopia: The mean difference between the two eyes was +6.34 ± 0.71 D at day 0 and +0.27 ± 0.49 D 2 days later (P < .001, Fig. 1A). Specifically, the axial reduction caused by prior +7 D lens wear recovered by 46% (day 0 vs. day 2: −0.19 ± 0.03 mm vs. −0.09 ± 0.03 mm, P > .05, Fig. 1B), vitreous chamber depth recovered by 83% (−0.29 ± 0.03 mm vs. −0.05 ± 0.02 mm, P > .05, Fig. 1C), and previous choroidal thickening dissipated (+0.09 ± 0.02 mm vs. +0.01± 0.01 mm, P > .05, Fig. 1D).
In comparison, after the positive lenses were removed, chick eyes in the +7-dark group recovered from prior hyperopia by 60% after kept in darkness for 3 days: The mean difference between the two eyes was +7.08 ± 0.80 D at day 0 and +3.90 ± 0.75 D 3 days later (P = .066, Fig. 1A). Indeed, all treated eyes (8 out of 8) developed a myopic shift in dark recovery conditions. The axial reduction recovered by 42% (day 0 vs. day 2, −0.20 ± 0.03 mm vs. −0.12 ± 0.04mm, P > .05, Fig. 1B) and the vitreous reduction recovered by 65% (−0.23 ± 0.02 mm vs. −0.08 ± 0.04 mm, P < .05, Fig. 1C). Prior choroidal thickening recovered by 70% (+0.12 ± 0.02 mm vs. +0.05 ± 0.01 mm, P > .05, Fig. 1D).
Recovery from Prior Negative Lens Wear
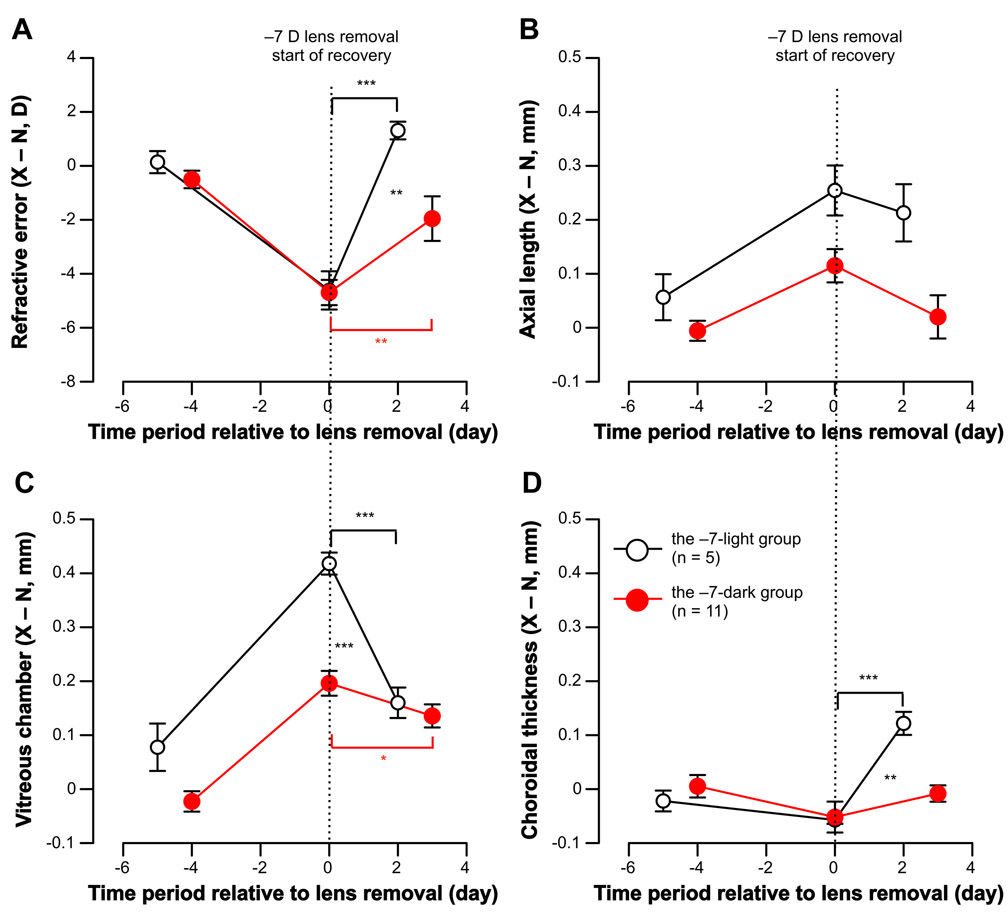
Wearing −7 D lenses over one eye for either 5 days (the −7-light group) or 4 days (the −7-dark group) caused robust compensation, and the lens-wearing eyes became significantly more myopic than their fellow eyes: The mean difference between the two eyes at the end of the lens-wear period (X – N) at day 0 was −4.62 (± 0.71) D for the −7-light group and −4.69 (± 0.47) D for the −7-dark group (P < .001 between the two eyes for both, Fig. 2A). This myopia development was caused by significantly increased axial length (the −7-light group: +0.25 ± 0.05 mm, the −7-dark group: +0.11 ± 0.03 mm; P < .001 for both, Fig. 2B) and vitreous chamber depth (the −7-light group: +0.42 ± 0.02 mm, the −7-dark group: +0.20 ± 0.02 mm; P < .001 for both, Fig. 2C), and significantly thinned choroids (the −7-light group: −0.06 ± 0.01 mm, the −7-dark group: −0.05 ± 0.03 mm; P < .05 for both, Fig. 2D). The inter-ocular difference at the end of lens wear (day 0) between these two groups was significantly different for axial length (P < .05, Fig. 2B) and vitreous chamber depth (P < .01, Fig. 2C).
Figure 2.
Comparison of recovery in the light and the dark after negative lens wear.Data is shown as the inter-ocular difference between the experimental and fellow eyes (X – N, Mean ± SEM) at various ages for (A) refractive error, (B) axial length, (C) vitreous chamber depth, and (D) choroidal thickness. Ages have been normalized so the day of lens removal (the start of recovery) is represented by zero on the X-axes. Asterisks with bars indicate statistically significant difference for inter-ocular difference (X – N) between various ages in each group (* p < .05, *** p < .001, One-Way Repeated Measures ANOVA), and asterisks without bars indicate statistically significant difference for relative difference (ΔX – ΔN) between the −7-light and −7-dark groups during recovery (** p < .01, *** p < .001, unpaired, 2-tailed Student’s t-test).
After the negative lenses were removed at day 0 for the −7-light group, chick eyes significantly recovered from prior myopia by 125% after staying in normal light for only 2 days (X – N at day 0 vs. day 2, −4.62 ± 0.71 D vs. +1.31 ± 0.33 D, P < .001, Table 2 and Fig. 2A). Specifically, the axial elongation caused by prior −7 D lens wear significantly recovered by 20% (+0.25 ± 0.05 mm vs. +0.21 ± 0.05 mm, P > .05, Fig. 2B), vitreous chamber depth recovered by 68% (+0.42 ± 0.02 mm vs. +0.16 ± 0.03 mm, P < .001, Fig. 2C). The choroids thickened 3 x greater than normal to recover from prior myopia (−0.06 ± 0.01 mm vs. +0.12 ± 0.02 mm, P < .001, Fig. 2D), which explained the rapid reduction in vitreous chamber depth.
In comparison, after the negative lenses were removed and animals raised in darkness for 3 days, chick eyes in the −7-dark group recovered from prior myopia by 69% (X – N at day 0 vs. day 3, −4.69 ± 0.47 D vs. −1.81 ± 0.56 D, P < .01, Table 2 and Fig. 2A). Specifically, all treated eyes (11 out of 11) developed a hyperopic shift. The axial elongation recovered by 75% (+0.11 ± 0.03 mm vs. +0.02 ± 0.04 mm, P = 0.51, Fig. 2B) and the vitreous elongation recovered by 27% (+0.20 ± 0.02 mm vs. +0.14 ± 0.02 mm, P < .05, Fig. 2C). Choroidal thinning recovered by 67% (−0.05 ± 0.03 mm vs. −0.01 ± 0.02 mm, P > .05, Fig. 2D).
DISCUSSION
The finding that chick eyes partially recovered from prior lens treatment while kept in darkness (in the absence of any visual input) support the existence of some factor not related to vision that appears to “know” the expected length of the eye.
The Effect of Dark Rearing
It may be thought that some of the recovery observed here is simply caused by darkness and a lack of light cycle per se. Previous studies have shown that constant darkness (from days, weeks, to months) caused excessive axial elongation and yet a hyperopic shift in monkeys,43-45 kittens,46 and chicks35, 47-49, the latter due to significant corneal flattening. It should be noted that the effects of dark rearing may differ between species, since it has been shown that tree shrews (on day 27 of visual experience, approximately 7 weeks of age) developed significant myopia (−4.3 ± 0.5 D) with elongated vitreous chamber depth (0.09 ± 0.02 mm) but no significant corneal flattening after 10 days of constant darkness.50
In chicks, the previously reported hyperopic shift with dark rearing is caused by corneal flattening.35, 48 For example, after 4 weeks of dark rearing, chick eyes developed hyperopia (+8.24 D), significantly different from normal age-matched controls (+2.60 D), and the vitreous chamber depth in these eyes were significantly longer than the age-matched normal animals (6.69 mm vs. 5.86 mm).35 The corneal curvature was significantly increased after both 2 and 6 weeks of dark rearing (after 2 weeks, 3.29 mm vs. 3.18 mm; after 6 weeks, 4.44 mm vs. 3.97 mm).48These effects are quite different to what we observe here. In the current study, where chick eyes recovered in darkness for only 2 to 3 days, hyperopic shifts after minus lens wear were accompanied by a decrease in eye length, and although corneal power was not measured, the anterior chamber depth was unaffected. Dark rearing effects are reported after longer periods than in the current study in which changes occurred after only a few days. Certainly, they are unlikely to explain the opposite changes that we observe with positive versus negative prior lens wear.
Perhaps more relevant, dark rearing has been reported to shift the diurnal rhythm in axial length in untreated chicks.49 While the rhythm in axial length still persisted while the chicks were kept in constant darkness for 4 days, the peak of the rhythm occurred slightly earlier than that of eyes raised in a normal 14:10 hour light:dark cycle, and the growth rate of the eye became significantly higher than normal eyes (117 vs. 72 μm / day). It is possible that such enhanced growth rates (predicted to be 117 μm over 24 hours versus 83 μm that we observe) may support the recovery from induced hyperopia after positive lens wear removal. However, if true, it suggests that the inhibited growth rate observed after negative lens-wear removal is an underestimate of the actual strength of the underlying inhibitory signal. Therefore, it is unlikely that chick eyes recovered from prior negative lens wear in darkness because of the effect dark rearing may have on the diurnal rhythm.
Regardless of the above effects, the fact that opposite changes in the direction of growth appropriate for the eye to regain a matched eye length with its untreated fellow eye, in the absence of any visual input, suggests that the eye growth control system has access to some kind of intrinsic homeostatic mechanism that either “knows” the expected absolute size of the eye for a particular age of development or is sensitive to differences between the two eyes.
Comparison with Previous Studies
The findings here are consistent with preliminary results in guinea pigs in which they were observed to recover from myopia induced by negative lens-wear after 3 days of darkness by slowing their vitreous chamber elongation.37 On the other hand, tree shrew eyes that were myopic as a result of prior lens treatment did not recover after the animals were kept in constant darkness for 10 days.50 However, there was a consistent myopic shift in both the treated and the untreated fellow eyes caused by axial elongation that was not offset by corneal flattening as occurs in chicks, monkeys, kittens and probably guinea pigs. This suggests that any recovery signals may have been overridden by this unusual darkness-induced myopic offset. These different results could also be attributed to differences in the age of animals used in these two experiments: The guinea pigs were very young (13 days after birth) when the eyes were still actively emmetropizing (guinea pig eyes emmetropize since birth until at least 30 days of life;51 while the tree shrews were older (around 48 days old on average) when they were placed in darkness, and were in a relatively late stage of their emmetropization process at this age.50 Note that tree shrew eyes generally open at 3 weeks of age, and actively emmetropize from 2 weeks to 5 weeks after normal eye opening.50, 52 It is also possible that the non-visual factors are weaker or that there is a weaker drive to couple growth between the two eyes in tree shrews than in guinea pigs.50
Possible Molecular Pathway Responsible for the Non-visual Cue(s)
The normal size of a tissue or organ is maintained through a highly coordinated regulatory process of cell growth, proliferation, and apoptosis.53 It has been suggested that the size of a tissue or organ itself is regulated, independent of the cell size, cellular growth rate, and the environment in which the tissue or organ is grown, i.e., the size information is intrinsic to the tissue itself (a more detailed review can been seen in Crickmore and Mann (2008)54). Cell competition is an example of this intrinsic mechanism. Several candidate processes and signals have also been suggested to be potential contributors, such as transforming growth factor-beta 1.55 While it is not clear exactly what parameters are used to determine final organ size, previous studies argue against the simple “cell counting” or “amount of time spent growing” models of size regulation.54 When it comes to the eye, even though it is speculated that non-visual, intrinsic mechanisms are sensitive to the size or shape of the eye, it is important to note that the actual mechanism does not necessarily only detect axial length to determine the correct eye size. Indeed, it may use any physical or chemical signals, such as pressure and temperature within the tissue.
The Hippo pathway has been shown to be a master regulator for size-determining purposes,53 and it is possible that it is involved in eye grow regulation. Several studies have shown that the Hippo pathway plays an important role in the eye in various species: (1) The Hippo pathway effector YAP controls frog retinal stem cell DNA replication time and genomic stability,56 (2) the Hippo pathway effector Yki downregulates Wg signaling to promote retinal differentiation in the Drosophila eye,57 (3) the Hippo pathway controls a switch between retinal progenitor cell proliferation and photoreceptor cell differentiation in Zebrafish.58 The Hippo pathway has also been shown to regulate the retinal pigment epithelium proliferation and differentiation. Mutation of the Hippo pathway, on the other hand, has been shown to cause abnormal eye growth. Overexpression of Yki phenocopies increases proliferation, defective apoptosis, and tissue overgrowth in Drosophila.59 Further experiments are needed to further study the effect of the Hippo pathway in eye growth and its potential in reducing myopia progression.
Study Limitations
Chicks were various ages at the start of the experiment, ranging from 1 to 7 days of age. Since untreated chicks emmetropize most rapidly within the first 3 days of life, and only minor, nonsignificant changes in refractive error occur thereafter,60 it is possible that chicks that started lens wear at 7 days of age (the +7-light, +7-dark, and −7-dark groups) compensated less than those started at 1 day of age (the −7-light group). However, it is unlikely that this limitation significantly affected the interpretation of the results, since chicks in the −7-light and −7-dark groups showed very similar amounts of compensation for −7 D lenses before recovery, in terms of refractive error (ΔX – ΔN during the course of −7 D lens wear, the −7-light group vs. the −7-dark group, −4.79 ± 0.45 D vs. −4.19 ± 0.48 D; P = .44, 2-tailed, unpaired, Student’s t-test), axial length (0.20 ± 0.04 mm vs. 0.12 ± 0.04 mm; P = .23), and choroidal thickness (−0.04 ± 0.03 mm vs. −0.06 ± 0.04 mm; P = .74). On the other hand, chicks in the −7-light group showed more vitreous chamber elongation compared with those in the −7-dark group (0.34 ± 0.05 mm vs. 0.22 ± 0.03 mm; P = .0178), possibly caused by the early starting age. In addition, during recovery from prior −7 D lens wear, chicks in the −7-light group showed a significantly larger hyperopic shift and more vitreous chamber inhibition compared with those in the −7-dark group, facilitated by choroidal thickening during recovery from prior negative lens wear under light (the −7-light group, Fig. 2). It is possible that this difference in response was caused by the different starting ages. However, it has been shown that untreated chick eyes grow in a linear fashion by an average of 72 μm per day.42
A second limitation was that lens removal also took place at various days of age (14 days of age for the +7-light group, 11 days of age for the +7-dark and −7-dark groups, and 6 days of age for the −7-light group), and the lens treatment durations were different for positive and negative lens treatment (e.g., chicks in the +7-light and +7-dark groups wore +7 D lenses for 7 and 4 days, respectively). It is plausible that the difference in these parameters may potentially cause different magnitudes in both lens compensation and recovery from prior lens treatment. However, it is unlikely the case, since chick eyes in all four groups completely and accurately compensated for both +7 D and −7 D lenses, regardless of these differences. Experiments were designed so that they occurred when chicks were between 1 to 14 days of age, during which chick eyes rapidly emmetropize.61 Taken together, it is unlikely that the different starting ages and lens treatment durations can explain the results observed.
The third limitation was the difference in the recovery period under light (2 days) and in darkness (3 days). However, it is unlikely that these time differences caused the substantially different amounts of recovery in light versus in darkness. Indeed, if a longer recovery period causes a larger amount of recovery, chick eyes recovered in darkness would have shown a larger amount of recovery compared with those recovered under light, which is contradictory to our findings.
The fourth limitation was that chicks may not have been observed long enough while they recovered in darkness (the +7-dark and −7-dark groups). Chick eyes only recovered from prior positive and negative lens treatment by 60% and 69% after 3 days of dark rearing, respectively. Since the experiments were terminated at this point, it is unknown whether chick eyes can fully recovery from prior lens treatment. Therefore, the effect of the proposed non-visual mechanism(s) in guiding the magnitude of eye growth was not studied in this paper. It would be interesting to keep chicks in darkness for longer durations to study if the eyes can completely recovery from prior lens without any visual input.
Finally, it may be thought that short periods of exposure to light during the measurement process could underlie the recovery in the dark. However, several results argue against this possibility. First, all measures were done with absolutely minimal exposure to low light. Second, based on temporal properties of lens wear, at least 1.5 mins of defocus repeated every hour is required for light signal to summate.39 In addition, chicks were anaesthetized during both measures, and it has been shown that chick eyes did not respond to defocus during anesthesia.62 Never-the-less, it is acknowledged that the question of what the state of consciousness or what state the retina needs to be to respond to visual signals which control eye growth is not yet elucidated.
In summary, even though there are timing differences in the experimental design, these differences do not impact the finding that chick eyes partially recover from prior lens treatment in a bidirectional manner when recovered in darkness.
Implications for Human Myopia Control
This study suggests that some intrinsic, non-visual factors or mechanisms can guide eye growth to recover from prior lens treatment in the absence of any visual input, as if the eyes try to return to their individual set-point refractions during recovery as previously suggested.63 It is possible that these mechanisms are sensitive to recent asymmetric changes in eye length or size and can guide eye growth in the absence of any visual input. It is not clear whether these intrinsic factors are only sensitive to asymmetric changes or if they are also sensitive to changes in eye length or size. We speculate that if the intrinsic factor is sensitive to asymmetry between the two eyes in their size and/or shape (implying a sort of “cross-talk” between both eyes), it may be able to guide ametropic eyes to equalize their size in darkness as shown here, but may fail to facilitate recovery if both eyes were abnormally sized. This could be tested using additional treatment groups in which both eyes would be covered with lenses prior to dark rearing.
This non-visual mechanism seems to at least exist in chick eyes. Whether or not it exits in mammalian eye is still debatable, since although guinea pig eyes have been shown to recover from prior negative lens wear and form deprivation,37 tree shrew eyes do not recover from −5D lenses in the dark, although as noted above, tree-shew eyes have a different response to darkness than other mammalian species.50 Myopia progression in school-aged children has been suggested to be a failure of regulated eye growth64, since once the eye becomes myopic, the myopic defocus in front of the retina (when uncorrected) should prevent further axial elongation.65 If the basis of this intrinsic mechanism is non-visual, and the same mechanism exists in humans, it could have the potential to reduce myopic development and/or prevention in school-aged children.
The main approach for current myopia control is to utilize optical devices (e.g., bifocals, progressive additional lenses, multi-zone contact lenses, and orthokeratology) to superimpose myopic defocus onto the (primarily peripheral) retina to slow myopia progression. None of these treatment options completely stops myopic progression. For those that do slow down myopia progression in the first a couple of years, the therapeutic effect slowly diminishes in the following years (more detailed reviews on myopia control can be seen in Huang et al. (2016)66 and Wildsoet et al. (2019)67). One of the reasons that these optical treatments are not 100% effective might be that they do not address possible non-visual mechanisms that might also be involved in eye growth regulation. Consistent with findings in this paper, it has been shown in chick eyes that there seems to be an endogenous, possibly genetic, determination of the set-point of emmetropization63, e.g., the end-point of refractive error in the eye might be partially determined by some unknown endogenous non-visual mechanism. If the exact genes or molecular cascades that are involved in the proposed intrinsic mechanisms could be identified, perhaps non-optical therapies could be developed to enhance the actions of these genes or molecular cascades to prevent myopia progression. Furthermore, one might also expect that myopia control could be optimized if the proposed potential non-optical therapy could be combined with optical treatment(s).
CONCLUSIONS
We show that the chick eye can recover bi-directionally from lens induced defocus in the dark without a visual signal. This suggests that anisometropic chick eyes can use an intrinsic, homeostatic mechanism to guide the eye to regain their normal size. Since eye growth control is phylogenetically conserved across many species, it is possible that non-visual factor(s) may contribute more generally to emmetropization and is not exclusively based on a local visual feedback system.
ACKNOWLEDGMENTS
Supported by: NIH EY02727 (Josh Wallman).
The authors sincerely thank the late Josh Wallman for his contribution to this project and dedicate this publication to him.
Contributor Information
Xiaoying Zhu, College of Optometry, State University of New York, New York, New York.
Sally A. McFadden, School of Psychology, Faculty of Science, University of Newcastle, Callaghan, New South Wales, Australia.
REFERENCES
- 1.Troilo D, Smith EL 3rd, Nickla DL, et al. IMI - Report on Experimental Models of Emmetropization and Myopia. Invest Ophthalmol Vis Sci 2019;60:M31–M88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallman J, Winawer J. Homeostasis of Eye Growth and the Question of Myopia. Neuron 2004;43:447–68. [DOI] [PubMed] [Google Scholar]
- 3.Kroger RH, Wagner HJ. The Eye of the Blue Acara (Aequidens pulcher, Cichlidae) Grows to Compensate for Defocus Due to Chromatic Aberration. J Comp Physiol (A) 1996;179:837–42. [DOI] [PubMed] [Google Scholar]
- 4.Kroger RH, Hirt B, Wagner HJ. Effects of Retinal Dopamine Depletion on the Growth of the Fish Eye. J Comp Physiol (A) 1999;184:403–12. [DOI] [PubMed] [Google Scholar]
- 5.Irving EL, Sivak JG, Callender MG. Refractive Plasticity of the Developing Chick Eye. Ophthalmic Physiol Opt 1992;12:448–56. [PubMed] [Google Scholar]
- 6.Schaeffel F, Glasser A, Howland HC. Accommodation, Refractive Error and Eye Growth in Chickens. Vision Res 1988;28:639–57. [DOI] [PubMed] [Google Scholar]
- 7.Hung LF, Crawford ML, Smith EL. Spectacle Lenses Alter Eye Growth and the Refractive Status of Young Monkeys. Nature Med 1995;1:761–5. [DOI] [PubMed] [Google Scholar]
- 8.Graham B, Judge SJ. The Effects of Spectacle Wear in Infancy on Eye Growth and Refractive Error in the Marmoset (Callithrix Jacchus). Vision Res 1999;39:189–206. [DOI] [PubMed] [Google Scholar]
- 9.Siegwart JT Jr., Norton TT. Refractive and Ocular Changes in Tree Shrews Raised with Plus or Minus Lenses. Invest Ophthalmol Vis Sci 1993;34:1208. [Google Scholar]
- 10.McFadden SA, Howlett MH, Mertz JR, Wallman J. Acute Effects of Dietary Retinoic Acid on Ocular Components in the Growing Chick. Exp Eye Res 2006;83:949–61. [DOI] [PubMed] [Google Scholar]
- 11.Barathi VA, Boopathi VG, Yap EP, Beuerman RW. Two Models of Experimental Myopia in the Mouse. Vision Res 2008;48:904–16. [DOI] [PubMed] [Google Scholar]
- 12.Wildsoet C, Wallman J. Choroidal and Scleral Mechanisms of Compensation for Spectacle Lenses in Chicks. Vision Res 1995;35:1175–94. [DOI] [PubMed] [Google Scholar]
- 13.Wallman J, Wildsoet C, Xu A, et al. Moving the Retina: Choroidal Modulation of Refractive State. Vision Res 1995;35:37–50. [DOI] [PubMed] [Google Scholar]
- 14.Siegwart JT Jr., Norton TT. The Susceptible Period for Deprivation-Induced Myopia in Tree Shrew. Vision Res 1998;38:3505–15. [DOI] [PubMed] [Google Scholar]
- 15.Troilo D, Nickla DL, Wildsoet CF. Choroidal Thickness Changes During Altered Eye Growth and Refractive State in a Primate. Invest Ophthalmol Vis Sci 2000;41:1249–58. [PubMed] [Google Scholar]
- 16.Hung LF, Wallman J, Smith EL 3rd, . Vision-Dependent Changes in the Choroidal Thickness of Macaque Monkeys. Invest Ophthalmol Vis Sci 2000;41:1259–69. [PubMed] [Google Scholar]
- 17.Howlett MH, McFadden SA. Form-Deprivation Myopia in the Guinea Pig (Cavia porcellus). Vision Res 2006;46:267–83. [DOI] [PubMed] [Google Scholar]
- 18.Howlett MH, McFadden SA. Spectacle Lens Compensation in the Pigmented Guinea Pig. Vision Res 2009;49:219–27. [DOI] [PubMed] [Google Scholar]
- 19.Chakraborty R, Read SA, Collins MJ. Monocular Myopic Defocus and Daily Changes in Axial Length and Choroidal Thickness of Human Eyes. Exp Eye Res 2012;103:47–54. [DOI] [PubMed] [Google Scholar]
- 20.Chakraborty R, Read SA, Collins MJ. Hyperopic Defocus and Diurnal Changes in Human Choroid and Axial Length. Optom Vis Sci 2013;90:1187–98. [DOI] [PubMed] [Google Scholar]
- 21.Chiang ST, Phillips JR, Backhouse S. Effect of Retinal Image Defocus on the Thickness of the Human Choroid. Ophthalmic Physiol Opt 2015;35:405–13. [DOI] [PubMed] [Google Scholar]
- 22.Wang D, Chun RK, Liu M, et al. Optical Defocus Rapidly Changes Choroidal Thickness in Schoolchildren. PLoS One 2016;11:e0161535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sherman SM, Norton TT, Casagrande VA. Myopia in the Lid-Sutured Tree Shrew (Tupaia glis). Brain Res 1977;124:154–7. [DOI] [PubMed] [Google Scholar]
- 24.Wiesel TN, Raviola E. Myopia and Eye Enlargement after Neonatal Lid Fusion in Monkeys. Nature 1977;266:66–8. [DOI] [PubMed] [Google Scholar]
- 25.von Noorden GK, Crawford ML. Lid Closure and Refractive Error in Macaque Monkeys. Nature 1978;272:53–4. [DOI] [PubMed] [Google Scholar]
- 26.Troilo D, Judge SJ. Ocular Development and Visual Deprivation Myopia in the Common Marmoset (Callithrix jacchus). Vision Res 1993;33:1311–24. [DOI] [PubMed] [Google Scholar]
- 27.Whatham AR, Judge SJ. Compensatory Changes in Eye Growth and Refraction Induced by Daily Wear of Soft Contact Lenses in Young Marmosets. Vision Res 2001;41:267–73. [DOI] [PubMed] [Google Scholar]
- 28.McFadden SA, Howlett MH, Mertz JR. Retinoic Acid Signals the Direction of Ocular Elongation in the Guinea Pig Eye. Vision Res 2004;44:643–53. [DOI] [PubMed] [Google Scholar]
- 29.Tejedor J, de la Villa P. Refractive Changes Induced by Form Deprivation in the Mouse Eye. Invest Ophthalmol Vis Sci 2003;44:32–6. [DOI] [PubMed] [Google Scholar]
- 30.Schaeffel F, Burkhardt E, Howland HC, Williams RW. Measurement of Refractive State and Deprivation Myopia in Two Strains of Mice. Optom Vis Sci 2004;81:99–110. [DOI] [PubMed] [Google Scholar]
- 31.Zhu X, Park TW, Winawer J, Wallman J. In a Matter of Minutes, the Eye Can Know Which Way to Grow. Invest Ophthalmol Vis Sci 2005;46:2238–41. [DOI] [PubMed] [Google Scholar]
- 32.Rucker FJ. The Role of Luminance and Chromatic Cues in Emmetropisation. Ophthalmic Physiol Opt 2013;33:196–214. [DOI] [PubMed] [Google Scholar]
- 33.Charman WN. Aberrations and Myopia. Ophthalmic Physiol Opt 2005;25:285–301. [DOI] [PubMed] [Google Scholar]
- 34.Kee CS. Astigmatism and Its Role in Emmetropization. Exp Eye Res 2013;114:89–95. [DOI] [PubMed] [Google Scholar]
- 35.Troilo D, Wallman J. The Regulation of Eye Growth and Refractive State: An Experimental Study of Emmetropization. Vision Res 1991;31:1237–50. [DOI] [PubMed] [Google Scholar]
- 36.Schaeffel F, Howland HC. Properties of the Feedback Loops Controlling Eye Growth and Refractive State in the Chicken. Vision Res 1991;31:717–34. [DOI] [PubMed] [Google Scholar]
- 37.McFadden S, Hawkins N, Howlett MH. Recovery from Experimentally Induced Myopia in the Guinea Pig. Exp Eye Res. 2004;79:92. [Google Scholar]
- 38.Xu A Local Choroidal and Scleral Mechanisms of Recovery from Partial Myopia in Chicks. PhD dissertation. The City University of New York; 1992. [Google Scholar]
- 39.Zhu X, Wallman J. Temporal Properties of Compensation for Positive and Negative Spectacle Lenses in Chicks. Invest Ophthalmol Vis Sci 2009;50:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallman J, Adams JI. Developmental Aspects of Experimental Myopia in Chicks: Susceptibility, Recovery and Relation to Emmetropization. Vision Res 1987;27:1139–63. [DOI] [PubMed] [Google Scholar]
- 41.Nickla DL. Diurnal Rythms and Eye Growth in Chicks. PhD dissertation. City University of New York; 1996. [Google Scholar]
- 42.Nickla DL, Wildsoet C, Wallman J. Visual Influences on Diurnal Rhythms in Ocular Length and Choroidal Thickness in Chick Eyes. Exp Eye Res 1998;66:163–81. [DOI] [PubMed] [Google Scholar]
- 43.Regal DM, Boothe R, Teller DY, Sackett GP. Visual Acuity and Visual Responsiveness in Dark-Reared Monkeys (Macaca nemestrina). Vision Res 1976;16:523–30. [DOI] [PubMed] [Google Scholar]
- 44.Raviola E, Wiesel TN. Effect of Dark-Rearing on Experimental Myopia in Monkeys. Invest Ophthalmol Vis Sci 1978;17:485–8. [PubMed] [Google Scholar]
- 45.Guyton DL, Greene PR, Scholz RT. Dark-Rearing Interference with Emmetropization in the Rhesus Monkey. Invest Ophthalmol Vis Sci 1989;30:761–4. [PubMed] [Google Scholar]
- 46.Yinon U, Koslowe KC. Eyelid Closure Effects on the Refractive Error of the Eye in Dark- and in Light-Reared Kittens. Am J Optom Physiol Opt 1984;61:271–3. [DOI] [PubMed] [Google Scholar]
- 47.Yinon U, Koslowe KC. Hypermetropia in Dark Reared Chicks and the Effect of Lid Suture. Vision Res 1986;26:999–1005. [DOI] [PubMed] [Google Scholar]
- 48.Gottlieb MD, Fugate-Wentzek LA, Wallman J. Different Visual Deprivations Produce Different Ametropias and Different Eye Shapes. Invest Ophthalmol Vis Sci 1987;28:1225–35. [PubMed] [Google Scholar]
- 49.Nickla DL, Wildsoet CF, Troilo D. Endogenous Rhythms in Axial Length and Choroidal Thickness in Chicks: Implications for Ocular Growth Regulation. Invest Ophthalmol Vis Sci 2001;42:584–8. [PubMed] [Google Scholar]
- 50.Norton TT, Amedo AO, Siegwart JT Jr., Darkness Causes Myopia in Visually Experienced Tree Shrews. Invest Ophthalmol Vis Sci 2006;47:4700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howlett MH, McFadden SA. Emmetropization and Schematic Eye Models in Developing Pigmented Guinea Pigs. Vision Res 2007;47:1178–90. [DOI] [PubMed] [Google Scholar]
- 52.Norton TT, Amedo AO, Siegwart JT Jr., The Effect of Age on Compensation for a Negative Lens and Recovery from Lens-Induced Myopia in Tree Shrews (Tupaia glis belangeri). Vision Res 2010;50:564–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pan D Hippo Signaling in Organ Size Control. Genes Devel 2007;21:886–97. [DOI] [PubMed] [Google Scholar]
- 54.Crickmore MA, Mann RS. The Control of Size in Animals: Insights from Selector Genes. Bioessays 2008;30:843–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michalopoulos GK, DeFrances M. Liver Regeneration. Adv Biochem Eng Biotechnol 2005;93:101–34. [DOI] [PubMed] [Google Scholar]
- 56.Cabochette P, Vega-Lopez G, Bitard J, et al. YAP Controls Retinal Stem Cell DNA Replication Timing and Genomic Stability. Elife 2015;4:e08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wittkorn E, Sarkar A, Garcia K, et al. The Hippo Pathway Effector YKI Downregulates WG Signaling to Promote Retinal Differentiation in the Drosophila Eye. Development 2015;142:2002–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asaoka Y, Hata S, Namae M, et al. The Hippo Pathway Controls a Switch between Retinal Progenitor Cell Proliferation and Photoreceptor Cell Differentiation in Zebrafish. PLoS One 2014;9:e97365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang J, Wu S, Barrera J, et al. The Hippo Signaling Pathway Coordinately Regulates Cell Proliferation and Apoptosis by Inactivating Yorkie, the Drosophila Homolog of YAP. Cell 2005;122:421–34. [DOI] [PubMed] [Google Scholar]
- 60.Avila NV, McFadden SA. A Detailed Paraxial Schematic Eye for the White Leghorn Chick. J Comp Physiol (A) 2010;196:825–40. [DOI] [PubMed] [Google Scholar]
- 61.Schaeffel F, Feldkaemper M. Animal Models in Myopia Research. Clin Exp Optom 2015;98:507–17. [DOI] [PubMed] [Google Scholar]
- 62.Bitzer M, Schaeffel F. Zenk Expression of Retinal Glucagon Amacrine Cells in Chicks: The Effect of Defocus Presented in Vivo, in Vitro and under Anesthesia. Vision Res 2006;46:848–59. [DOI] [PubMed] [Google Scholar]
- 63.Tepelus TC, Schaeffel F. Individual Set-Point and Gain of Emmetropization in Chickens. Vision Res 2010;50:57–64. [DOI] [PubMed] [Google Scholar]
- 64.Flitcroft DI. Is Myopia a Failure of Homeostasis? Exp Eye Res 2013;114:16–24. [DOI] [PubMed] [Google Scholar]
- 65.Zhu X, Winawer JA, Wallman J. Potency of Myopic Defocus in Spectacle Lens Compensation. Invest Ophthalmol Vis Sci 2003;44:2818–27. [DOI] [PubMed] [Google Scholar]
- 66.Huang J, Wen D, Wang Q, et al. Efficacy Comparison of 16 Interventions for Myopia Control in Children: A Network Meta-Analysis. Ophthalmology 2016;123:697–708. [DOI] [PubMed] [Google Scholar]
- 67.Wildsoet CF, Chia A, Cho P, et al. Imi - Interventions Myopia Institute: Interventions for Controlling Myopia Onset and Progression Report. Invest Ophthalmol Vis Sci 2019;60:M106–M31. [DOI] [PubMed] [Google Scholar]