Figure 3. FOXO1 Inhibition Prevents Latency Establishment and Reactivates HIV in Primary CD4+ T cells and HIV-infected CD4+ T cells.
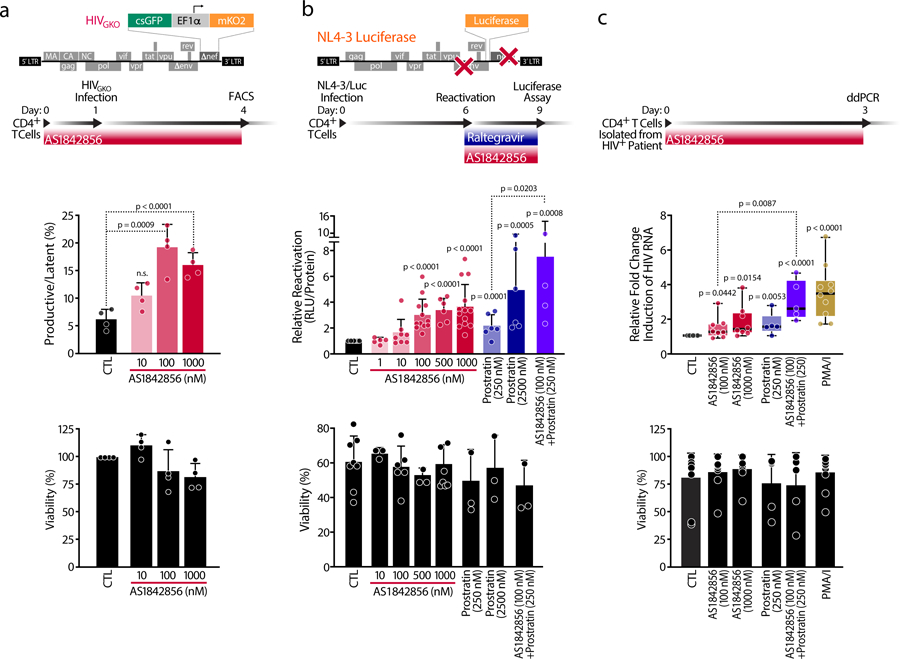
a, Schematic representation of HIVGKO dual-labeled HIV-1 reporter and of strategy used to treat primaryCD4+ T cells purified from blood of healthy donors and pre-treated for 24 hours with increasing concentrations of AS1842856. After infection of HIVGKO, resting cells were treated for 3 days with the same amounts of AS1842856 as in the pre-treatment. Top panel shows ratios of productive versus latent populations of infected CD4+ T cells from four different healthy donors after treatment. Lower panel shows a histogram plot of percent live cells for each drug treatment relative to the control. Data are represented by mean ± SD of n=4 different donors. b, Schematic representation of HIVNL4–3 Luciferase reporter virus and of experimental procedure with primary CD4+ T cells. Briefly, CD4+ T cells were purified from blood of healthy donors, were infected with HIVNL4–3 Luciferase and, after 6 days, the virus was reactivated for 3 days with increasing concentrations of AS1842856 and prostratin alone or in combination, in the presence of raltegravir (30 μM). HIV reactivation was measured by luciferase activity and cell viability by flow cytometry. Data are mean ± SEM of n≥5 individual donors. c, Fold change of cell-associated HIV-1 mRNA expression measured by ddPCR of CD4+ T cells of HIV-infected patients on antiretroviral-therapy with undetectable viral load treated with 50 ng/ml PMA + 1 μM Ionomycin (PMA/I) and with 0.2 % DMSO (CTL). AS1842856- and/or prostratin-treated CD4+ T cells led to an increase in fold change of cell-associated HIV mRNA expression. Data are represented as box plots of n≥5 independent experiments. Cell viability was assessed by Tripan Blue exclusion. Data are represented as mean ± SD of n≥5 independent experiments.
