Figure 6. Induction of ER stress promotes HIV reactivation.
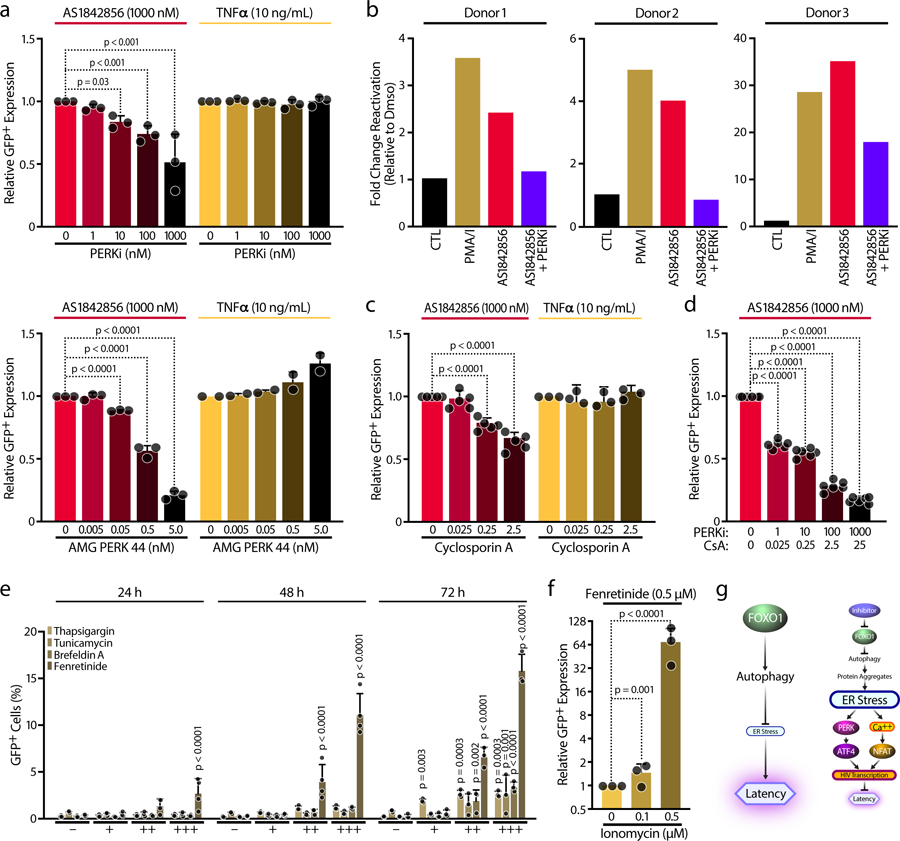
a, J-Lat cell line 5A8 were treated with increasing concentrations of PERKi (GSK2656157 / PERK inhibitor II) (top panels) or the highly specific PERK inhibitor (AMG PERK 44) (lower panels) and co-treated with 1,000 nM AS1842856 (72 hours) or 10 ng/mL TNFα (24 hours). HIV-GFP reactivation was analyzed by FACS and normalized to the control. Data shown are mean ± SD of n=3 independent experiments. b, Effect of 50 ng/ml PMA + 1 μM Ionomycin (PMA/I), AS1842856 (1,000 nM) and AS1842856 (1,000 nM) with 1 μM PERKi (GSK2656157 / PERK inhibitor II) on HIV-1 mRNA expression in CD4+ T cells of n=3 HIV-infected patients on antiretroviral-therapy with undetectable viral load measured by ddPCR. c, Same experiment as in Fig. 6a, but cells were treated with increasing concentrations of Cyclosporin A (CsA). d, Similar experiment that in Fig. 5b or Fig. 5c, but combining increasing concentrations of PERKi (GSK2656157 / PERK inhibitor II) and Cyclosporin A (CsA). Data represent mean ± SD of n=6 independent experiments. e, J-Lat cell line A58 was treated with increasing concentrations of Thapsigargin (0.01, 0.1, 1 µM), Tunicamycin (0.1, 0.5, 1 µg/mL), Brefeldin A (0.01, 0.1, 1 µg/mL) and Fenretinide (0.5, 2, 5 µM) for 24, 48 and 72 hours and HIV-GFP reactivation was analyzed by FACS. Data shown are mean ± SD of n≥3 independent experiments. f, J-Lat cell line A58 was treated with 0.5 µM Fenretinide and increasing concentrations of Ionomycin. HIV-GFP reactivation and cell viability were analyzed by FACS. Data are mean ± SD of n=3 independent experiments. g, Model: FOXO1 inhibition impairs autophagy, thus promoting protein accumulation and leading to ER Stress. Thus, ATF4 activation through PERK and NFAT via cytosolic calcium release will promote HIV transcription and will prevent HIV latency.
