Figure 7.
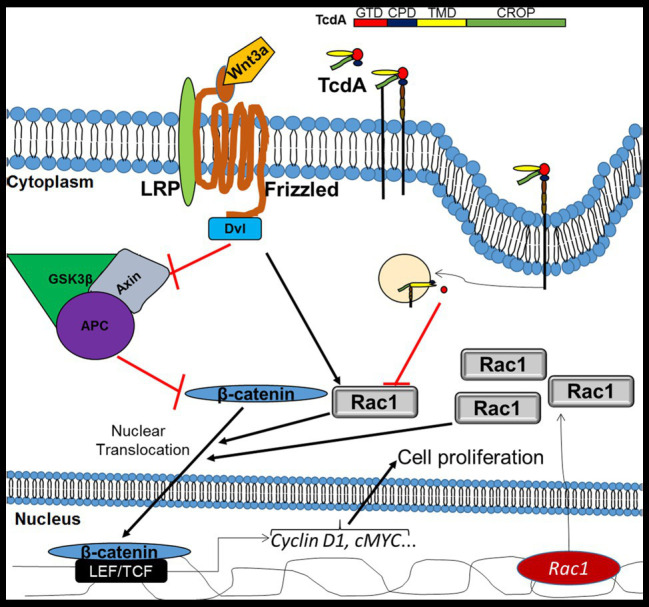
Mechanism of inhibition of Wnt/β-catenin signaling by TcdA. In IECs, Wnt3a binds to the frizzled (FZD) receptor and its coreceptor lymphoid enhancer factor (LRP), recruiting disheveled (Dvl), which in turn inhibits the adenomatous polyposis coli (APC)/glycogen synthase kinase 3 beta (GSK3β)/Axin complex, which degrade β-catenin. Upon inhibition of the APC/GSK3β/APC complex, β-catenin accumulates in the cytoplasm and is translocated into the nucleus, with Rac1 serving as a promoter of this translocation, where it activates LEF/TCF to promote the transcription of target genes, such as cyclin D1 and cMYC. Both cyclin D1 and cMYC are involved in the induction of cell proliferation. TcdA [composed of glucosyltransferase domain (GDT), cysteine protease domain (CPD), translocation membrane domain (TMD), and C-terminal combined repetitive oligopeptide (CROP)] binds to a cell receptor by CROP and is endocytosed. Then, GDT is released into the cytosol, where it inhibits Rac1, resulting in a decrease in β-catenin translocation to the nucleus with a consequent reduction in the genic expression of the target genes and cell proliferation. However, upregulation of Rac1, in the presence of Wnt3a activity, decreases TcdA-induced inhibition of Wnt3a/β-catenin signaling and proliferation. APC, adenomatous polyposis coli; CPD, cysteine protease domain; CROPs, C-terminal combined repetitive oligopeptides; Dvl, disheveled; GSK3β, glycogen synthase kinase 3 beta; GTD, glucosyltransferase domain; LEF, lymphoid enhancer factor; LRP, low-density-lipoprotein-related protein; TCF, T cell factor protein; and TMD, translocation membrane domain.
