Abstract
Early detection will be crucial for effective treatment or prevention of Alzheimer’s disease. The identification and validation of early, non-invasive biomarkers is therefore key to avoiding the most devastating aspects of Alzheimer’s. Measures of central auditory processing such as gap detection have recently emerged as potential biomarkers in both human patients and the 5XFAD mouse model of Alzheimer’s. Full validation of gap detection deficits as a biomarker will require detailed understanding of the underlying neuropathology, including which brain structures are involved and how the operation of neural circuits is affected. Here we show that 5XFAD mice exhibit gap detection deficits as early as 2 months of age, well before development of Alzheimer’s-associated pathology. We then examined responses of neurons in auditory cortex to gaps in white noise. Both gap responses and baseline firing rates were robustly and progressively degraded in 5XFAD mice compared to littermate controls. These impairments were first evident at 2–4 months of age in males, and 4–6 months in females. This demonstrates early-onset impairments to the central auditory system, which could be due to damage in auditory cortex, upstream subcortical structures, or both.
INTRODUCTION
Among the difficulties faced by Alzheimer’s patients is a decline in communication skills. The nature of this decline is distinct from presbycusis, a common form of age-related hearing loss that typically affects higher frequencies and is generally associated with damage to peripheral structures such as the cochlea (for review, see Gates & Mills, 2005; Ohlemiller & Gagnon, 2004). In contrast, Alzheimer’s patients have impaired temporal processing and speech perception, which are hallmarks of central auditory dysfunction (Gates, Anderson, McCurry, Feeney, & Larson, 2011; Haggstrom, Rosenhall, Hederstierna, Ostberg, & Idrizbegovic, 2018; Idrizbegovic et al., 2011). Alzheimer’s-associated pathology has been described in several brain regions involved in auditory processing (Sinha, Hollen, Rodriguez, & Miller, 1993). However, auditory impairments precede the development of full-blown Alzheimer’s disease (AD), and therefore may be valid AD predictors (Gates, Beiser, Rees, D’Agostino, & Wolf, 2002; Tuwaig et al., 2017). There is a growing consensus that effective treatment or prevention of Alzheimer’s disease will require intervention well before the appearance of cognitive impairments (Jack & Holtzman, 2013). Specific auditory temporal processing deficits and central auditory dysfunction may therefore help identify at-risk populations for therapeutic intervention prior to the development of irreversible AD pathology and cognitive decline (Bidelman, Lowther, Tak, & Alain, 2017; Gates et al., 2002; Tuwaig et al., 2017).
Gap detection is a test of temporal acuity that is well-established across a wide range of species from mice to humans (for example, see Ison, O’Connor, Bowen, & Bocirnea, 1991; Okanoya & Dooling, 1990; Plomp, 1964). We measured gap detection using a common variant of pre-pulse inhibition of the acoustic startle response, in which a silent gap inserted into continuous background noise acts as a cue that attenuates the startle reflex. The longer the gap, the greater the attenuation. Detection of brief gaps (≤ 32 ms duration) requires processing in auditory cortex (Ison et al., 1991; Threlkeld, Penley, Rosen, & Fitch, 2008; Weible, Moore, et al., 2014; Weible, Yavorska, & Wehr, 2020), whereas detection of longer gaps can be mediated by auditory brainstem and midbrain structures. Thus the pattern of impairments across gap durations may shed light on where dysfunction occurs along the central auditory pathway.
Gap detection deficits are correlated with speech perception deficits in older adults (Fitzgibbons & Gordon-Salant, 1996; Glasberg, Moore, & Bacon, 1987; Snell & Frisina, 2000). Importantly, gap detection deficits are also seen in patients diagnosed with Mild Cognitive Impairment (MCI; Iliadou et al., 2017). While not all those diagnosed with MCI go on to develop Alzheimer’s disease, it is viewed as an intermediate stage between normal, age-matched cognitive function and Alzheimer’s (Bidelman et al., 2017; Swords, Nguyen, Mudar, & Llano, 2018). Gap detection tests may therefore prove useful in the diagnosis of pre-clinical AD in the absence of cognitive deficits. To have maximal value as a biomarker, however, it will be critical to understand the neuropathology underlying the gap detection deficits. Biomarker validity is highest when there is a full biological understanding of the relationship between a biomarker and disease. In addition, understanding why and how gap detection is impaired in AD may improve early detection by targeting non-invasive measures of neural function, as well as provide a basis for potential therapeutic approaches. Mouse models of AD may be especially useful here, by enabling detailed investigation of the molecular and circuit pathology underlying biomarkers such as auditory temporal processing deficits.
We recently demonstrated early-onset gap detection deficits in the 5XFAD mouse model of AD (Kaylegian, Stebritz, Weible, & Wehr, 2019). The 5XFAD mouse co-expresses five familial Alzheimer’s disease mutations, and has proven to be a valuable model organism for studying AD due to its rapid recapitulation of many hallmarks of the disease (Oakley et al., 2006). Significant deficits in gap detection occurred by 2 months of age, and were robust in both females and males compared with sex- and age-matched littermate controls (Kaylegian et al., 2019). This corresponds to an age when amyloid deposition has only just begun (Eimer & Vassar, 2013; Oakley et al., 2006), is 1–4 months before the first signs of structural pathology (Buskila, Crowe, & Ellis-Davies, 2013; Crowe & Ellis-Davies, 2013, 2014; Jawhar, Trawicka, Jenneckens, Bayer, & Wirths, 2012), and is at least 4 months before the first evidence of cell death (Eimer & Vassar, 2013). This is also 2–4 months before evidence of impaired cognitive and mnemonic processes associated with hippocampal dysfunction in these mice (Devi & Ohno, 2010; Jawhar et al., 2012; Oakley et al., 2006; Ohno, 2009; Ohno et al., 2006). This latter point is especially important as deficits in hippocampus-based learning and memory are those that present most dramatically in AD patients. Together these findings clearly support the use of gap detection as a key metric for identifying central auditory processing deficits associated with prodromal AD, as well as the utility of the 5XFAD mouse for studying circuit mechanisms underlying Alzheimer’s-related temporal processing deficits.
Here we examined behavioral and auditory cortical neuron responses to gap-in-noise stimuli in 5XFAD mice ranging from 1 to 6 months of age. Gap detection behavior recapitulated our previous findings: 5XFAD mice showed significant deficits by 2 months of age, across the full range of gap durations in both females and males (Kaylegian et al., 2019). Gap responses of auditory cortical neurons in mice less than 2 months of age were largely similar for both female and male 5XFAD mice relative to controls. With age, however, deficits emerged. Gap responses were significantly decreased across all gap durations, and baseline firing rates were lower, in 5XFAD mice relative to controls. While it is not yet clear precisely how these differences relate to the observed impairments in behavior, these results appear to indicate changes in both cortical and subcortical processing of auditory information.
METHODS
All procedures were in accordance with the National Institutes of Health guidelines, as approved by the University of Oregon Animal Care and Use Committee.
Mice.
All mice were 4–12 weeks of age at the time of surgery. Mice were heterozygous for 5XFAD (n = 14 mice, 7 females and 7 males, stock number 006554, The Jackson Laboratory) on a C57BL6/SJ1 hybrid background (stock number 100012, The Jackson Laboratory), with wildtype littermates as controls (n = 13 C57BL6/SJ1 mice, 6 females and 7 males). This background is heterozygous for the retinal degeneration mutation Pde6brd1, which causes blindness in Pde6brd1 homozygous mice; we therefore excluded offspring homozygous for Pde6brd1 from this study.
Surgery.
We administered atropine (0.03 mg/kg) pre-operatively to reduce inflammation and respiratory irregularities. Surgical anesthesia was maintained with isoflurane (1.25–2.0%). For recording single neurons in auditory cortex, an array of 8 tetrodes was inserted vertically through a small craniotomy (1 mm × 0.8 mm) dorsal to auditory cortex, and cemented into place with Grip Cement (Dentsply, Milford, DE). Ketoprofen (4.0 mg/kg) was administered post-operatively to minimize discomfort. Mice were housed individually after the surgery and allowed 7 days of post-operative recovery.
Stimulus delivery.
All data were collected in a sound-attenuating chamber. Sounds were delivered from a free-field speaker directly facing the animal. The speaker was calibrated to within ±1 dB using a Brüel and Kjær 4939 1/4-inch microphone positioned where the ear would be but without the animal present. Mice were loosely restrained in a plastic tube (35 mm inner diameter, 1.5 mm wall thickness) affixed to a flat base. The head was fixed in position. The tube was perforated (~3 mm diameter) to allow effective transmission of sound, with no more than 5 dB attenuation. An open slot along the top enabled access to the implanted fibers. To measure the startle response, the tube rested on a piezo transducer. Movement signals from the piezo transducer were amplified and digitized at 10 kHz.
We measured behavioral gap detection using a variant of pre-pulse inhibition of the acoustic startle response, in which a gap that precedes a startle stimulus acts as a cue that reduces the magnitude of the startle response. Acoustic stimuli were embedded in continuous background white noise (80 dB SPL). Startle stimuli (25ms white noise bursts, 100 dB SPL) were separated by a random inter-trial interval of 15 ± 5 s. Silent gaps in the continuous background noise preceded the startle stimulus, separated by a 50 ms interval between the end of the gap and the onset of the startle stimulus. Gap durations were 1, 2, 3, 4, 8, 32, or 256 ms, with 20 presentations per behavioral session, and did not include ramps at onset or offset. We also presented pure startle stimuli in isolation, without a gap (the “gap-free” condition). We separately characterized neural spiking responses to a more comprehensive range of gap durations (1, 2, 3, 4, 8, 16, 32, 64, 128 or 256 ms), as well as gap-free trials, 20 repetitions, with a 1 s ITI and no startle stimulus.
Single neuron recording.
We recorded from single neurons using an implanted array of 8 tetrodes passed through a 26-gauge stainless steel hypodermic tube. Tetrodes were made of 18 μm (25 μm coated) tungsten wire (California Fine Wire). The entire array was mounted on a custom microdrive. Tetrode data were acquired with 32-channel RHD2000 hardware (Intan Technologies) and Open Ephys software (http://open-ephys.org). A minimum threshold of 50 μV was set for collection of spiking activity. Spiking activity of individual neurons was isolated offline using the open source spike sorting software packages Simpleclust (Voigts, 2013) and MClust (Redish, 2008). Measures of peak and trough waveform voltage, energy, and principal components analysis were used as waveform separation parameters in 2-dimensional cluster space. Cells were accepted for analysis only if they had a cluster boundary completely separate from adjacent cluster boundaries, and completely above threshold, on at least one 2-D view. Additionally, cells with events during a 2 ms refractory window in the interspike interval histogram ≥ 0.5% of the total spike count were excluded from analysis.
Gap termination responses (GTRs) and rate decreasing responses (RDRs) were identified as significant increases or decreases, respectively, in the average firing rate during the 50 ms post-gap interval, compared to an equivalent interval during background noise on gap-free trials (paired t-test). Off responses were identified as significant increases in average firing rate during a window following sound offset (i.e., the start of the gap) compared to the corresponding window on gap-free trials. For 128 ms & 256 ms durations, the Off response window was 75 ms. For 32 ms and 64 ms gaps, we used the full duration of the gap and a comparable window from gap-free trials. We did not measure Off responses for gaps shorter than 32 ms. For each response type, a cell was only considered to have a response if that response was significant for two consecutive gap durations, for robustness. We categorized cells according to their gap responses as either GTR cells, RDR cells, or Off cells. A few cells had more than one type of response (e.g., 1.6% of cells showed both a GTR for some gap durations and an RDR for others). For simplicity, we categorized any cell exhibiting both a GTR and an RDR as a GTR cell (see Fig. 3D&E for examples). Categorizing them instead as RDR cells or excluding them from analysis had negligible effects on the results. Any cell with Off responses was categorized as an Off responsive cell, and if it also showed a GTR or RDR it was also included in those categories.
Figure 3. Examples of gap responses in auditory cortical neurons.

We categorized cells into 3 types based on their gap responses.
A) A Gap Termination Response (GTR) is a burst of firing following the end of the gap, a type of On response (black arrow). Cells with GTRs were categorized as GTR cells. Gaps are indicated by gray shading. Each row is a different gap duration (indicated at left in ms), aligned to gap termination. This example cell showed only a GTR.
B) This cell showed both an Off response to the beginning of the silent gap (e.g., grey arrow), and a GTR to the resumption of noise at the end of the gap (e.g., black arrow). This cell was categorized as both an Off cell and a GTR cell.
C) This cell showed an Off response, and was categorized as an Off cell. Because the Off response carried over into the post-gap interval (black arrow), this cell was also categorized as a GTR cell.
D) This cell showed both a GTR (black arrow) and significantly suppressed firing during the post-gap interval with longer gap durations (asterisk). Because of the GTR, this cell was categorized as a GTR cell and not a Rate Decreasing Response (RDR) cell.
E) This cell showed significantly suppressed firing during the post-gap interval (asterisk) and was therefore categorized as an RDR cell.
Data analysis.
For behavioral sessions, we quantified startle responses by calculating the peak of the rectified startle response signal in a 100 ms window following startle stimulus onset. We quantified gap detection as the percent reduction in the median startle response compared to the median gap-free startle response for each mouse. In some cases, startle responses were slightly facilitated following a gap (e.g., for 1 ms gaps), which resulted in a negative gap detection value. Mice were tested approximately once per week from ages 37 to 176 days (a period of 20 weeks), although not all mice were tested at all age points (average: 6.8 sessions per mouse, range: 1–19). To determine at which age the behavioral gap detection deficits in 5XFAD mice were first detectable, we used a 1-tailed Wilcoxon rank-sum test on 256 ms gap detection in age bins increasing in 5 day increments (e.g., 37–45 days, 37–50 days, 37–55 days, etc.). This test showed no difference earlier than 37–60 days and a significant difference for all bins 37–60 days and longer, from which we conclude that the deficit in 5XFAD mice is first detectable at 60 days (Kaylegian et al., 2019).
Analyses of behavioral and single-neuron spiking data were performed using non-parametric tests because some of the comparisons involved non-normally distributed data (Lilliefors test), and because statistical power was comparable even when the underlying assumptions for the corresponding parametric analysis were met (Kitchen, 2009). We used the Kruskal-Wallis test (non-parametric alternative to the 1-way Anova) to assess group differences between 5XFAD and control mice across gap durations, as well as within-group changes across age ranges. Where the Kruskal-Wallis test returned a significant main effect, we used the Wilcoxon rank-sum test (non-parametric alternative to the unpaired t-test) for post-hoc comparisons of individual gap durations. To identify differences in pure startle amplitude and baseline firing rate, we used the rank-sum test on data from gap-free trials. We report effect sizes as eta-squared (η2) (Lenhard & Lenhard, 2016). η2 varies between 0 and 1, and corresponds to the proportion of variance in the dependent variable explained by the independent variable. η2 values of 0.01 – 0.06 are generally considered to be small effects, η2 of 0.06 – 0.14 moderate effects, and η2 > 0.14 large effects. We used Matlab for all data analysis and statistics. All figures show group data as medians. Error bars show IQR, which is generally asymmetric (because the 25th and 75th percentiles are not necessarily symmetric about the median).
Histology.
To verify that recordings were in auditory cortex, we coronally sectioned all brains at 100 μm. Sections were mounted to slides and cover-slipped with DAPI Fluoromount-G (SouthernBiotech, #0100–20). Sections exhibiting evidence of tetrode tracks were photomicrographed and matched to atlas sections (Paxinos & Franklin 2001). We verified that recording tetrodes accurately targeted auditory cortex using the structure of the hippocampus and the rhinal fissure as rostrocaudal and dorsoventral landmarks. Only activity from tetrodes histologically verified as being from auditory cortex (areas Au1, AuV, and AuD in Paxinos & Franklin, 2001) were included in the analyses of neuronal data.
RESULTS
Behavior
We measured behavioral gap detection in mice using a variant of pre-pulse inhibition in which a silent gap in continuous background noise acts as a cue that reduces the acoustic startle response. 5XFAD mice showed robust and progressive gap detection deficits, confirming previous findings (Kaylegian et al., 2019). Gap detection in 5XFAD mice was significantly worse than controls (Figure 1A&B, p = 7.8 × 10−38 , η2 = 0.18, Kruskal-Wallis, 5XFAD: n = 83 sessions from 13 mice, 6 females and 7 males; control: n = 94 sessions from 13 mice, 7 females and 6 males), and this difference was detectable by post-natal day 60 (p = 0.01, Kruskal-Wallis).
Figure 1. Behavioral gap detection is impaired in 5XFAD mice.
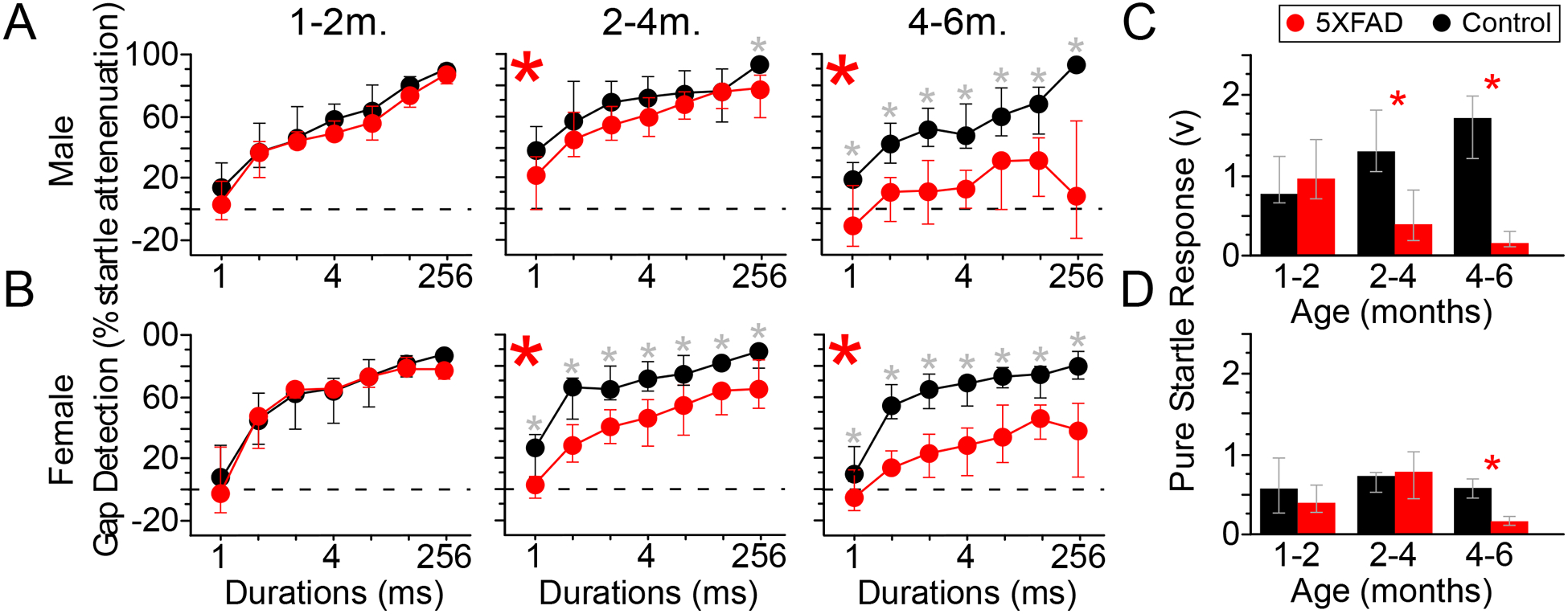
5XFAD mice first showed impaired gap detection at approximately 2 months of age.
A, B) Gap detection was impaired in both male and female 5XFAD mice relative to controls, beginning at 2–4 months of age. Red: 5XFAD, black: control. Red asterisks indicate significant overall effects (Kruskal-Wallis), grey asterisks indicate significant effects at individual gap durations (post-hoc).
C, D) At 1–2 months of age (1–2m), pure startle responses were no different between 5XFAD mice and sex-matched controls (C: males, D: females). Red: 5XFAD, black: control. Beginning at 2–4 months (2–4m), pure startle responses were smaller in male 5XFAD mice relative to controls, and in female 5XFAD mice relative to controls beginning at 4–6 months of age (4–6m). Startle stimuli were 100 dB white noise, presented during 80 dB background noise.
Red asterisks indicate significant differences between 5XFAD and control (rank-sum). Values are medians, error bars are IQR.
To examine how performance varied by sex and age, we separately analyzed male and female mice at three age ranges: ≤ 60 days (1–2 months old or “1–2m”), 61–120 days (2–4 months old or “2–4m”) and >120 days (4–6 months old or “4–6m”). Both male and female 5XFAD mice showed robust and progressive gap detection deficits (Fig. 1A&B). Gap detection by both male and female 5XFAD mice declined steadily over time (p = 1.1 × 10−19, η2 = 0.65; p = 6.9 × 10−7, η2 = 0.86, respectively, Kruskal-Wallis). These changes over time resulted in significantly worse gap detection by both male and female 5XFAD mice relative to controls at 2–4 months (males p = 0.0004, η2 = 0.08; females p = 6.2 × 10−12, η2 = 0.22, Kruskal-Wallis) and 4–6 months (males p = 2.2 × 10−18, η2 = 0.4; females p = 3.9 × 10−14, η2 = 0.37, Kruskal-Wallis). Female 5XFAD mice performed more poorly than males at 2–4 months (p = 0.0007, η2 = 0.05, Kruskal-Wallis), but at 4–6 months this was reversed, with male 5XFAD mice performing worse than females (p = 0.0003, η2 = 0.06, Kruskal-Wallis). No sex-linked differences were observed for controls at any of the three age ranges. Pure startle responses (i.e., startles on gap-free trials) were also affected. At 1–2 months, male and female 5XFAD mice had pure startle responses that were comparable to those of controls, but startle responses then decreased with age (Fig. 1D&E; male 2–4m, p = 0.0001, η2 = 0.4; male 4–6m, p = 3.1 × 10−6, η2 = 0.7; female 4–6m, p = 2.5 × 10−6, η2 = 0.72, rank-sum).
Neuronal Activity
These gap detection deficits raise the possibility that processing might be impaired in central auditory structures. We therefore examined neural responses to gaps in auditory cortex. We recorded from 1646 cortical neurons, using tetrodes histologically verified to have been in auditory cortex (Fig. 2; 5XFAD female: 6 mice, 348 cells; 5XFAD male: 5 mice, 374 cells; control female: 6 mice, 563 cells; control male: 5 mice, 361 cells). We categorized cells based on their gap responses (see Table 1). Some cells showed Gap Termination Responses (GTRs), which we defined as a significant increase in firing during the post-gap interval, whereas other cells showed Rate Decreasing Responses (RDRs), a significant decrease in firing during the post-gap interval. Some cells also showed Off responses, which we defined as significantly increased firing following the start of the gap itself. We consider each of these response types below.
Figure 2. Recorded neurons were in auditory cortex.

All recordings were from tetrodes histologically verified as having been within auditory cortex (Au1, AuD, or AuV). Anterior-Posterior (AP) coordinates are relative to bregma. AuD: dorsal auditory subfield; AuV: ventral auditory subfield; rf: rhinal fissure.
Table 1.
Response Types by Genotype and Gender (1–2m, 2–4m, 4–6m)
| Genotype | Sex | Number of cells with GTRs | Number of cells with Off responses | Number of cells with RDRs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5XFAD | Female: 348 cells | 21 | 60 | 57 | 17 | 33 | 28 | 3 | 28 | 23 |
| Male: 374 cells | 58 | 15 | 20 | 31 | 11 | 14 | 7 | 6 | 23 | |
| Control | Female: 563 cells | 61 | 31 | 47 | 31 | 26 | 29 | 14 | 13 | 34 |
| Male: 361 cells | 31 | 31 | 48 | 17 | 17 | 29 | 19 | 34 | 40 | |
The proportion of cells that responded to gaps was different between 5XFAD mice and controls, with males and females showing opposite effects. Taking all response types together (GTRs, RDRs, and Off responses), there were significantly fewer responsive cells in male 5XFAD mice (38%) than in controls (58%) (χ2(1,735) = 30.9, p < 0.0001, see Table 1). In females, this was reversed, with more responsive cells in 5XFAD mice (59%) than in controls (39%) (χ2(1,911) = 33.0, p < 0.0001, see Table 1).
Gap Termination Responses
GTRs are a type of “On” response, evoked by the resumption of noise at the end of the gap. The overall response profiles of GTR cells were heterogeneous. Some cells responded strictly during the post-gap interval (e.g., Fig. 3A), while others responded to both noise offset and onset (i.e., Off responses and GTRs; Fig. 3B). A small subset of GTRs appeared to be strictly Off responses that carried over into the post-gap interval with shorter gap durations (e.g., Fig. 3C). In a few cases, GTR cells also showed a significant reduction during the post-gap interval with longer gap durations (e.g., Fig. 3D); we classified these as GTR cells rather than RDR cells (Fig. 3E shows an example of an RDR cell). For simplicity, here we consider GTR cells as a single population.
Both baseline and evoked firing rates in GTR cells were progressively and robustly decreased in 5XFAD mice (Fig. 4). Baseline firing rates were significantly lower for both 5XFAD males and females starting at 4–6 months (Fig. 4A, 4–6m, males: p = 0.0001, η2 = 0.21, females: p = 0.01, η2 = 0.06, rank-sum). GTRs for 5XFAD mice first showed a slight but significant increase, followed by a strong decrease (Fig. 4B). In males, GTRs first slightly increased at 1–2 months (p = 0.009, η2 = 0.007, Kruskal-Wallis), but then strongly decreased at 2–4 and 4–6 months (2–4m: p = 2.9 × 10−20, η2 = 0.16; 4–6m: p = 2.7 × 10−42, η2 = 0.26, Kruskal-Wallis). In females this sequence occurred later, with GTRs increased at 2–4 months, followed by a decrease at 4–6 months (2–4m: p = 0.01, η2 = 0.006, 4–6m: p = 0.01, η2 = 0.006, Kruskal-Wallis). In males, GTRs were affected across a broad range of gap durations (Fig. 4C), whereas for females GTRs were only affected for a subset of brief gaps (<32 ms in duration, Fig. 4D). These differences in spiking activity are generally consistent with the impairments we saw in gap detection behavior (Fig. 1), except that behavior in females was affected prior to GTRs.
Figure 4. Gap termination response (GTR) cells from older 5XFAD mice have lower firing rates than controls.
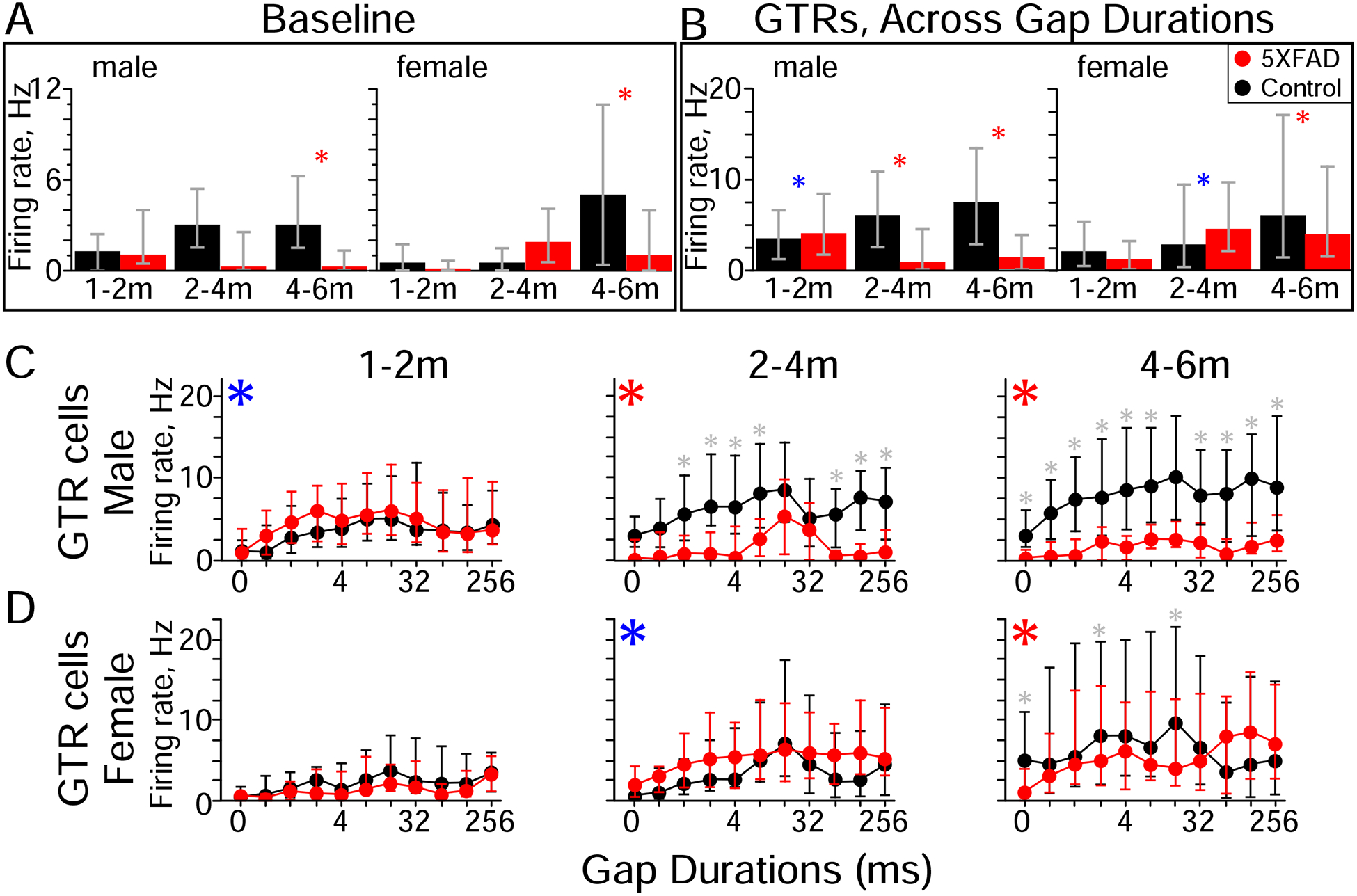
A) At 4–6 months of age, baseline firing rates of GTR cells from both male and female 5XFAD mice were significantly lower than those of sex-matched controls. Red asterisks indicate significant differences between 5XFAD and control (rank-sum). Baseline firing rates were measured during background noise in the absence of a gap. Red: 5XFAD, black: control.
B) GTRs in 5XFAD mice from both sexes showed a slight increase followed by a strong decrease compared to controls. In cells from 5XFAD males the decrease was evident at 2–4 months of age, and from females at 4–6 months of age. Blue asterisks indicate a significant increase in 5XFAD GTRs compared to controls, red asterisks indicate a decrease (Kruskal-Wallis).
C) GTRs were reduced across gap durations in male 5XFAD mice. Blue asterisks indicate a significant overall increase in 5XFAD GTRs compared to controls, red asterisks indicate a decrease (Kruskal-Wallis, same as B), grey asterisks indicate significant effects at individual gap durations (post-hoc). Gap durations of 0 ms correspond to baseline firing rates.
D) GTRs were only reduced for brief gap durations in female 5XFAD mice. Values are medians, error bars are IQR.
The lower firing rates in 5XFAD mice compared to controls were due to two factors: age-related changes in 5XFAD mice, and an age-related increase in control mice. In 5XFAD mice, males showed an age-related decrease in both baseline firing rates (p = 0.04, η2 = 0.05, Kruskal-Wallis) and GTRs (p = 1.5 × 10−27, η2 = 0.12, Kruskal-Wallis). In contrast, female 5XFAD mice showed an age-related increase in both baseline firing rates (p = 0.005, η2 = 0.06, Kruskal-Wallis) and GTRs (p = 2.1 × 10−20, η2 = 0.06, Kruskal-Wallis). In control mice, there was an overall increase with age for both baseline firing rates (males, p = 0.001, η2 = 0.1; females, p = 0.001, η2 = 0.08, Kruskal-Wallis) and GTRs (males, p = 1.4 × 10−18, η2 = 0.93; females, p = 4.4 × 10−13, η2 = 0.96, Kruskal-Wallis). In males, the age-related increases in controls and decreases in 5XFAD mice together produced a large net age-related decrease in GTRs in 5XFAD mice compared to controls (Fig. 4C). In females, the age-related increases in controls were greater than the age-related increases in 5XFAD mice, such that the net effect was a significant age-related decrease in 5XFAD mice compared to controls (Fig. 4D).
We wondered whether the decreases in GTRs and baseline firing rates in male 5XFAD mice were due to a single factor, manifesting as an overall loss of activity, or whether instead the drop in GTRs was a distinct effect, above and beyond the drop in baseline firing rates. To test this, we restricted analysis to only those cells with comparable baseline firing rates. To increase statistical power using this smaller data set, we combined cells from 2–4m and 4–6m male mice into a single “older” group. We used a range of ±1/2 the S.D. of the mean baseline of older male 5XFAD GTRs. Cells falling within this range (1.17 ± 0.98 Hz) still showed a significant difference in GTRs between 5XFADs and controls (p = 1.8 × 10−6, η2 = 0.06, Kruskal-Wallis), despite the relatively restricted sample size (5XFAD: 9 cells; control: 25 cells). We conclude that the age-related decrease in spiking activity in male 5XFAD mice is due to two independent factors: an overall drop in baseline firing rates, as well as a drop in gap-evoked GTRs.
Rate Decreasing Responses
An RDR is a gap-evoked suppression of firing rate below baseline. Like GTR cells, RDR cells showed an age-related decrease in activity in 5XFAD mice. At 1–2 months of age, RDR cells did not differ significantly by genotype for either baseline firing rates or RDRs for either sex (Fig. 5A&B). At 2–4 months, males and females showed different effects. RDR cells from 2–4 month old male 5XFAD mice had lower baseline firing rates (Fig. 5A, p = 0.001, η2 = 0.27, rank-sum) and RDRs (Fig. 5B, p = 1.0 × 10−10, η2 = 0.09, Kruskal-Wallis) compared to controls. In contrast, 2–4 month old female 5XFAD mice had slightly higher RDRs relative to controls (Fig. 5C, p = 0.01, η2 = 0.01, Kruskal-Wallis). At 4–6 months, baseline firing rates were reduced in males (Fig. 5A, p = 0.0004, η2 = 0.2, rank-sum), and RDRs in both male and female 5XFAD mice were lower than controls (males: p = 1.3 × 10−20, η2 = 0.13; females: p = 5.9 × 10−6, η2 = 0.03, Kruskal-Wallis). As with GTRs (Fig. 4), in males RDRs were affected across a broad range of gap durations (Fig. 5C), whereas for females RDRs were only affected for a subset of brief gaps (<32 ms in duration, Fig. 5D).
Figure 5. Rate decreasing response (RDR) cells from older 5XFAD mice had lower firing rates than controls.

A) Baseline firing rates of RDR cells were lower in older male 5XFAD mice, but unchanged in older female 5XFAD mice, relative to controls. Red asterisks indicate significant differences between 5XFAD and control (rank-sum). Red: 5XFAD, black: control.
B) RDR cells from both sexes generally showed reduced firing rates relative to controls. In cells from 5XFAD males this was evident at 2–4 months of age, and in females at 4–6 months of age. Red asterisks indicate a significant decrease in 5XFAD RDRs compared to controls, blue asterisks indicate an increase (Kruskal-Wallis).
C, D) By 4–6 months of age, the lower firing rate of RDR cells was seen across gap durations in male 5XFAD mice (C), but was restricted to brief gap durations (≤ 32 ms) in females (D). Red asterisks indicate a significant overall decrease in 5XFAD RDRs compared to controls, blue asterisks indicate an increase (Kruskal-Wallis, same as B), grey asterisks indicate significant effects at individual gap durations (post-hoc). Dashed lines indicate median baseline firing rate for cells from control (black) and 5XFAD (red) mice; note that rate-decreasing responses are suppressed below baseline. Values are medians, error bars are IQR.
Off Responses
An Off response is an increase in spiking immediately following offset of noise at the start of the gap (e.g., Fig. 3C). Some cells exhibiting Off responses also had significant spiking during the post-gap interval, and were thus also classified as GTR cells (e.g., Fig. 3B–D). Other cells exhibited an increase only during the gap itself. Thus the GTR and Off response populations were partially overlapping. Off responses at 1–2 months of age did not differ by genotype for either sex at baseline (Fig. 6A) or for evoked Off responses (Fig. 6B–D). Off responses from older male 5XFAD mice had significantly lower firing rates both at baseline (Fig. 6A, rank-sum, 4–6m, p = 0.0008, η2 = 0.26) and for evoked Off responses (Fig. 6B; Kruskal-Wallis, 2–4m, p = 0.0003, η2 = 0.11; 4–6m, p = 9.1 × 10−10, η2 = 0.22). This is consistent with the effects on firing rate seen with GTR cells. In contrast, Off responses in female 5XFAD mice were slightly higher at 2–4 months (Fig. 6D; Kruskal-Wallis, p = 0.02, η2 = 0.02).
Figure 6. Changes with age in Off response cell firing rate differ for male and female 5XFAD mice.
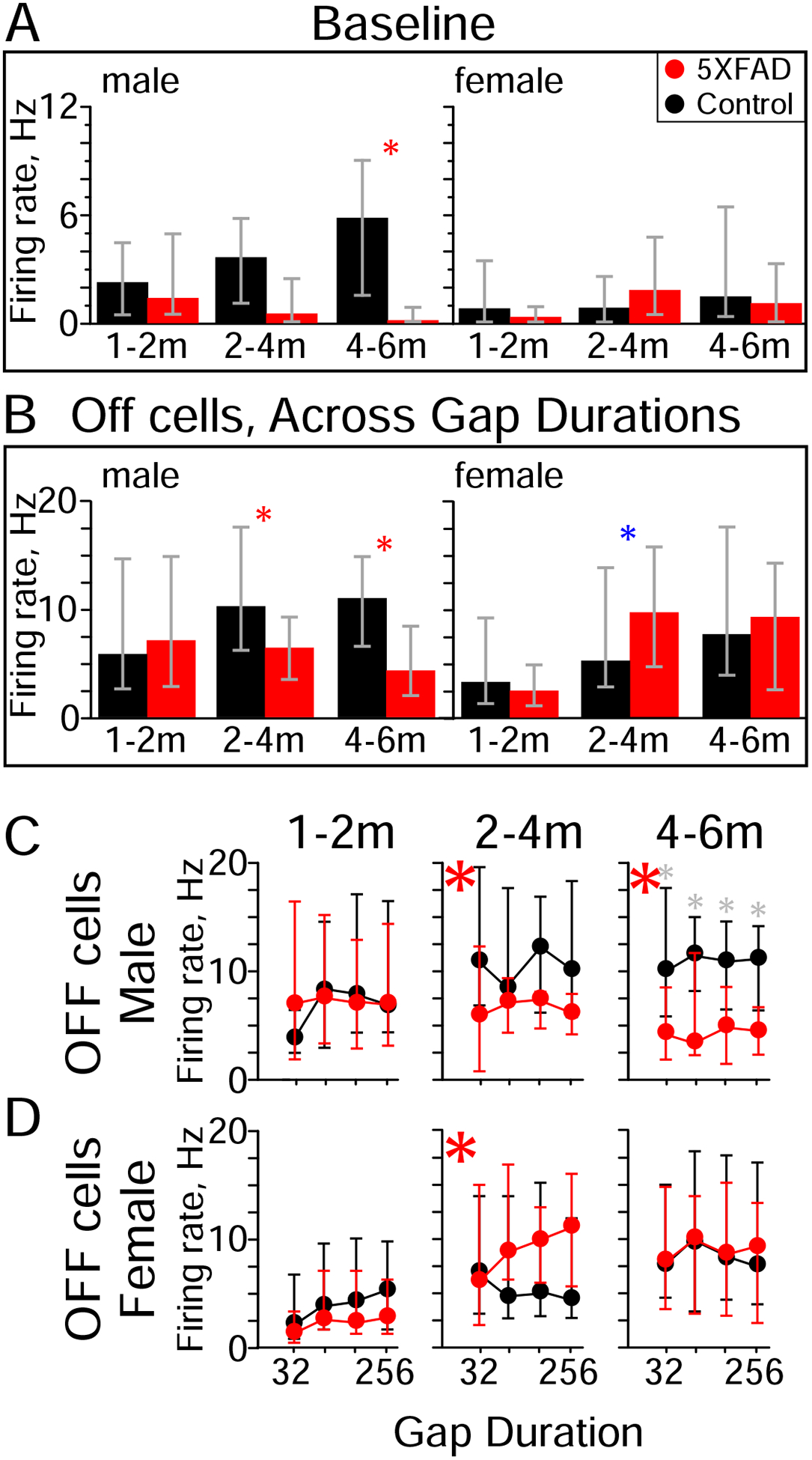
A) Baseline firing rates of Off response cells from male 5XFAD mice were significantly lower than controls at 4–6 months of age. No differences were seen with the same group of cells from female 5XFAD mice. Red asterisks indicate significant differences between 5XFAD and control (rank-sum). Red: 5XFAD, black: control.
B) Across gap durations, Off response cells from older male 5XFAD mice (2–4 and 4–6 months) had lower firing rates relative to controls. In contrast, Off response cells from female 5XFAD mice had higher firing rates at 2–4 months of age, and no difference at 4–6 months. Red asterisks indicate a significant overall decrease in 5XFAD Off responses compared to controls, blue asterisks indicate an increase (Kruskal-Wallis).
C, D) Post-hoc tests revealed that these differences only extended to individual gap durations for male 5XFAD mice at 4–6 months of age. Red asterisks indicate a significant overall decrease in 5XFAD Off responses compared to controls, blue asterisks indicate an increase (Kruskal-Wallis, same as B), grey asterisks indicate significant effects at individual gap durations (post-hoc). Values are medians, error bars are IQR.
Summary
Consistent with previous results, 5XFAD mice exhibited significantly worse gap detection beginning around 2 months of age. This impairment was evident in both males and females, and grew progressively worse with age. Baseline firing rates were significantly lower in 5XFAD mice, for all categories of cells. Gap-evoked responses were also significantly lower in 5XFAD mice compared to controls, both for cells with increased firing rates (GTRs) and decreased firing rates (RDRs). In males these differences appeared at age 2–4 months, and in females at 4–6 months.
DISCUSSION
Here we examined behavioral and neuronal responses to gap-in-noise stimuli in the 5XFAD mouse model of Alzheimer’s disease. Confirming previous results (Kaylegian et al., 2019), we found significant and progressive deficits in behavioral gap detection starting at 2 months of age in both male and female 5XFAD mice. In auditory cortex, neuronal activity of gap-responsive cells before 2 months of age was largely comparable between 5XFADs and controls of both sexes. Beyond 2 months of age, however, a number of differences developed. Neuronal gap termination responses from both male and female 5XFAD mice were markedly decreased, as were baseline firing rates. These differences were already evident in males at 2–4 months of age, and in females at 4–6 months. Cells with rate-decreasing responses showed a similar pattern of lower firing rates. Off responses during the gap were similarly decreased for males, but not for females. While it is not yet clear precisely how the decreased cortical firing rates relate to the behavioral deficits, our results clearly demonstrate profound changes in auditory cortex. These could be due to amyloid pathology in auditory cortex, could be inherited from upstream effects of amyloid pathology in subcortical structures, or both.
Auditory cortical gap responses are necessary for normal gap detection (Weible, Liu, Niell, & Wehr, 2014; Weible, Moore, et al., 2014; Weible et al., 2020). Optogenetic suppression of cortical gap responses impairs the detection of brief gaps (≤ 32 ms) in much the same way as conventional cortical lesions (Ison et al., 1991; Threlkeld et al., 2008; Weible et al., 2020). Responses to brief gaps are amplified in auditory cortex by the temporal summation of Off and On responses, resulting in a relatively large proportion of cortical neurons tuned for brief gap durations, whereas inferior colliculus neurons prefer longer gaps (Finlayson, 1999, 2002; Weible et al., 2020). Cortical suppression also reduces brief gap responses in inferior colliculus, indicating that under normal conditions corticofugal projections amplify collicular gap responses (Weible et al., 2020). Because inferior colliculus is involved in mediating pre-pulse inhibition (Koch, 1999; Parham & Willott, 1990), it seems likely that cortical GTRs impact gap detection behavior via this descending pathway. This suggests that the smaller GTRs seen in both male and female 5XFAD mice could explain their behavioral gap detection deficits. Our interpretation is that as cortical firing rates drop with age, cortex becomes less effective in driving gap detection behavior.
What might explain this difference in firing rate? One factor may be the direct impact of Aβ on neural activity. Extracellular Aβ aggregates are first detectable at 1.5 to 2 months of age (Eimer & Vassar, 2013; Macdonald et al., 2014; Oakley et al., 2006). Application of Aβ at low concentrations has been found to suppress spontaneous spiking activity (Nimmrich et al., 2008; Varghese et al., 2010). This indicates that the reduced baseline and evoked firing rates we observed in auditory cortical neurons may be directly tied to the early accumulation of extracellular Aβ. Other structural abnormalities may also contribute. One of the hallmarks of the 5XFAD mouse pathology is a pronounced loss of layer 5 pyramidal neurons (Eimer & Vassar, 2013; Jawhar et al., 2012; Oakley et al., 2006). While cell loss was only observed beginning at 9 months of age, axons of layer 5 cells showed evidence of dystrophy at 3–4 months of age (Crowe & Ellis-Davies, 2013; Jawhar et al., 2012), and physiological differences, such as reduced excitability, have been observed as early as 2–3 months (Buskila et al., 2013). Layer 5 provides the major corticofugal projection to inferior colliculus and other subcortical structures, as well as a projection to superficial layers (for review, see Adesnik & Naka, 2018; Bajo & King, 2012). Dysfunction in these cells could therefore contribute to lower firing rates not only in deep layers but across the cortical column. This could explain the overall drop in cortical firing rates with age, although a causal relationship has not been established.
Two observations suggest that subcortical dysfunction may also contribute to the gap detection deficits in 5XFAD mice. First, female 5XFAD mice had impaired gap detection at 2–4 months, prior to significant decreases in cortical firing rates at 4–6 months. Second, behavioral impairments in both sexes were seen following both brief and long gaps. Auditory cortex is critically involved in brief gap detection, but not the detection of longer gaps (Bowen, Lin, Taylor, & Ison, 2003; Ison et al., 1991; Kelly, Rooney, & Phillips, 1996; Threlkeld et al., 2008; Weible et al., 2020). This suggests that subcortical structures such as inferior colliculus are sufficient to mediate detection of long gaps, which in turn implicates subcortical damage in the impaired detection of long gaps by 5XFAD mice.
A recent study using a different Alzheimer’s mouse model (the APP/PS1 mouse) found evidence of hearing loss as early as 2 months of age (Liu et al., 2020). This hearing loss was manifested as a reduction in the magnitude of late components of the auditory brainstem response, ABR waves IV and V, which are thought to be generated by upper brainstem nuclei including the inferior colliculus and lateral lemniscus (Land, Burghard, & Kral, 2016). This provides further evidence of subcortical deficits in an Alzheimer’s mouse model, which if present in 5XFAD mice could also contribute to the gap detection deficits we observed.
Post-mortem analysis indicates only modest AD-associated pathology in auditory brainstem (Ohm & Braak, 1989), despite evidence of greater susceptibility of other brainstem nuclei to disease progression (for review, see Grinberg, Rueb, & Heinsen, 2011; see also Parvizi, Van Hoesen, & Damasio, 2001; Simic et al., 2009). However, patients given a probable AD diagnosis exhibit changes in brainstem auditory evoked potentials (Bidelman et al., 2017; Tachibana, Takeda, & Sugita, 1989), indicating AD-related changes at the earliest levels of auditory processing (but see Gates et al., 1995). Significant AD-related pathology has also been found in the inferior colliculus and the ventral nucleus of the medial geniculate (Lee, Ryan, Andreescu, Aizenstein, & Lim, 2015; Parvizi et al., 2001; Sinha et al., 1993). 5XFAD mice also show changes associated with AD pathology in each of these brain regions (Guzman et al., 2014; Oakley et al., 2006; Oh et al., 2018; Wirths et al., 2017). It therefore seems plausible that changes in behavioral gap detection may arise from both cortical and subcortical dysfunction.
The behavioral and neuronal changes we report here occur very early in the rapid and well-characterized progression of disease pathology in 5XFAD mice. Deficits in behavioral gap detection started around 2 months of age (this study, Kaylegian et al., 2019). Deficits in auditory cortical gap responses also appeared at 2–4 months. These effects coincide with the earliest reported changes associated with the pathological progression in the 5XFAD model. Intraneuronal and extracellular amyloid beta aggregates are first evident at 1.5 to 2 months of age (Eimer & Vassar, 2013; Macdonald et al., 2014; Oakley et al., 2006). Differential gene expression has been reported in the hippocampus of 5XFAD mice beginning around 2 months, and this increases dramatically by 4 months (Bundy, Vied, Badger, & Nowakowski, 2019; but see Mazi et al., 2018). Buskila and colleagues (2013) reported reduced excitability and other changes in pre- and post-synaptic function of layer 5 somatosensory neurons beginning around 2 months of age. Differences in metabolic activity across multiple brain regions were also detectable at this age, and were interpreted as evidence of decreased metabolic activity in cortex (Macdonald et al., 2014). By 4 months, distinct patterns of axonal dystrophy also occur (Crowe & Ellis-Davies, 2013). Importantly, the gap detection deficits we have reported here precede the earliest presentation of other behavioral/cognitive deficits in 5XFAD mice, including deficits in spatial and associative memory at 4–5 months (Girard et al., 2014; Oakley et al., 2006), motor dysfunction beginning at 6 months (Jawhar et al., 2012; O’Leary et al., 2017; Wagner et al., 2019), and contextual fear at 6–8 months (Kaczorowski, Sametsky, Shah, Vassar, & Disterhoft, 2011; Kimura & Ohno, 2009). As such, our data support the use of gap detection as an early behavioral and neurophysiological biomarker for AD. 5XFAD mice were also recently shown to have impaired visual responses, measured with retinal electrophysiology, as early as 1 month of age (Criscuolo et al., 2018). Together these results suggest that sensory systems may provide an early window into Alzheimer’s disease progression.
The extent to which sex is a critical factor when evaluating studies of 5XFAD data remains unclear, as many studies either use mice of only one sex, or do not include sex as a factor in their analyses. However, some sex-linked differences have been reported. 5XFAD transgenes are more highly expressed in female compared with male 5XFAD mice at 2 months and again at 4 months of age (Bundy et al., 2019). Levels of aromatase, the synthesizing enzyme for neuroprotective 17β-estradiol, is reduced in the hippocampus of female but not male 5XFAD mice compared with controls when measured at 3 months of age (Prange-Kiel et al., 2016). These results indicate more robust molecular pathology in females, and may shed light on the two-fold higher AD prevalence and faster cognitive decline in women (Andrew & Tierney, 2018; Ferretti et al., 2018; Mielke, 2018; Mielke, Vemuri, & Rocca, 2014). In the present study, behavioral gap detection in female 5XFAD mice was more impaired at 2–4 months relative to both controls and male 5XFAD mice, the same age as when other sex-linked differences in 5XFAD mice have been observed (Bundy et al., 2019; Prange-Kiel et al., 2016). However, by 4–6 months, male and female 5XFAD mice were equally impaired relative to controls, with 5XFAD males exhibiting a modestly greater deficit when compared directly to 5XFAD females. In contrast, electrophysiological deficits were greater and appeared earlier in male versus female 5XFAD cortical gap responses. Firing rates in males were strongly reduced by 2–4 months, but a clear difference in female 5XFAD mice versus controls only became apparent at 4–6 months. Male 5XFAD mice had fewer gap-responsive cells (and thus more non-responsive cells) than male controls, but notably, female 5XFAD mice had more gap-responsive cells than female controls. It is still unclear why cortical gap responses are affected earlier and more strongly in males, whereas behavioral gap detection deficits and molecular pathology are stronger and appear earlier in females. The absence of an obvious sex-linked difference in behavior at 4–6 months may reflect a floor effect of disease progression on gap detection, as both males and females are profoundly impaired. Moreover, the differences in cortical gap responses, as noted above, could reflect the combined downstream effects of pathology in multiple brain areas.
Alzheimer’s disease remains incurable. For any future treatment or therapy to be effective, it will need to be provided long before the onset of cognitive impairments, by which time extensive damage has already occurred. Thus early detection will be key to sparing Alzheimer’s patients from developing the more crippling cognitive impairments associated with the disease (Gates et al., 2002; Tuwaig et al., 2017). Here we have shown that behavioral gap detection is accompanied by profound dysfunction in central auditory processing, and these biomarkers occur well before the irreversible structural damage associated with AD. These findings support the potential use of gap detection deficits as an early biomarker of disease progression. This initial characterization of temporal processing deficits in auditory cortex lays the foundation for future studies of brainstem and midbrain structures of the central auditory system to further validate gap detection as an early signature of Alzheimer’s-related neural dysfunction.
Highlights.
5XFAD mice showed gap detection deficits as early as 2 months of age.
In auditory cortex, firing rates were robustly and progressively degraded.
These impairments were first evident at 2–4 months of age.
This demonstrates early-onset impairments to the central auditory system.
Acknowledgments
This work was supported by the National Institutes of Health National Institute on Deafness and Other Communication Disorders Grant R01 DC-015828.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors state that they have no conflicts of interest
REFERENCES
- Adesnik H, & Naka A (2018). Cracking the Function of Layers in the Sensory Cortex. Neuron, 100(5), 1028–1043. doi: 10.1016/j.neuron.2018.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew MK, & Tierney MC (2018). The puzzle of sex, gender and Alzheimer’s disease: Why are women more often affected than men? Women’s Health, 14, 1–8. doi: 10.1177/1745506518817995 [DOI] [Google Scholar]
- Bajo VM, & King AJ (2012). Cortical modulation of auditory processing in the midbrain. Front Neural Circuits, 6, 114. doi: 10.3389/fncir.2012.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidelman GM, Lowther JE, Tak SH, & Alain C (2017). Mild Cognitive Impairment Is Characterized by Deficient Brainstem and Cortical Representations of Speech. J Neurosci, 37(13), 3610–3620. doi: 10.1523/JNEUROSCI.3700-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen GP, Lin D, Taylor MK, & Ison JR (2003). Auditory cortex lesions in the rat impair both temporal acuity and noise increment thresholds, revealing a common neural substrate. Cereb Cortex, 13(8), 815–822. doi: 10.1093/cercor/13.8.815 [DOI] [PubMed] [Google Scholar]
- Bundy JL, Vied C, Badger C, & Nowakowski RS (2019). Sex-biased hippocampal pathology in the 5XFAD mouse model of Alzheimer’s disease: A multi-omic analysis. J Comp Neurol, 527(2), 462–475. doi: 10.1002/cne.24551 [DOI] [PubMed] [Google Scholar]
- Buskila Y, Crowe SE, & Ellis-Davies GC (2013). Synaptic deficits in layer 5 neurons precede overt structural decay in 5xFAD mice. Neuroscience, 254, 152–159. doi: 10.1016/j.neuroscience.2013.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscuolo C, Cerri E, Fabiani C, Capsoni S, Cattaneo A, & Domenici L (2018). The retina as a window to early dysfunctions of Alzheimer’s disease following studies with a 5xFAD mouse model. Neurobiol Aging, 67, 181–188. doi: 10.1016/j.neurobiolaging.2018.03.017 [DOI] [PubMed] [Google Scholar]
- Crowe SE, & Ellis-Davies GC (2013). In vivo characterization of a bigenic fluorescent mouse model of Alzheimer’s disease with neurodegeneration. J Comp Neurol, 521(10), 10.1002/cne.23336 [DOI] [PubMed] [Google Scholar]
- Crowe SE, & Ellis-Davies GC (2014). Spine pruning in 5xFAD mice starts on basal dendrites of layer 5 pyramidal neurons. Brain Struct Funct, 219(2), 571–580. doi: 10.1007/s00429-013-0518-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L, & Ohno M (2010). Genetic reductions of beta-site amyloid precursor protein-cleaving enzyme 1 and amyloid-beta ameliorate impairment of conditioned taste aversion memory in 5XFAD Alzheimer’s disease model mice. Eur J Neurosci, 31(1), 110–118. doi: 10.1111/j.1460-9568.2009.07031.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer WA, & Vassar R (2013). Neuron loss in the 5XFAD mouse model of Alzheimer’s disease correlates with intraneuronal Abeta42 accumulation and Caspase-3 activation. Mol Neurodegener, 8, 2. doi: 10.1186/1750-1326-8-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti MT, Iulita MF, Cavedo E, Chiesa PA, Schumacher Dimech A, Santuccione Chadha A, … the Alzheimer Precision Medicine, I. (2018). Sex differences in Alzheimer disease - the gateway to precision medicine. Nat Rev Neurol, 14(8), 457–469. doi: 10.1038/s41582-018-0032-9 [DOI] [PubMed] [Google Scholar]
- Finlayson PG (1999). Post-stimulatory suppression, facilitation and tuning for delays shape responses of inferior colliculus neurons to sequential pure tones. Hear Res, 131(1–2), 177–194. doi: 10.1016/s0378-5955(99)00032-5 [DOI] [PubMed] [Google Scholar]
- Finlayson PG (2002). Paired-tone stimuli reveal reductions and alterations in temporal processing in inferior colliculus neurons of aged animals. J Assoc Res Otolaryngol, 3(3), 321–331. doi: 10.1007/s101620020038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbons PJ, & Gordon-Salant S (1996). Auditory temporal processing in elderly listeners. J Am Acad Audiol, 7(3), 183–189. [PubMed] [Google Scholar]
- Gates GA, Anderson ML, McCurry SM, Feeney MP, & Larson EB (2011). Central auditory dysfunction as a harbinger of Alzheimer dementia. Arch Otolaryngol Head Neck Surg, 137(4), 390–395. doi: 10.1001/archoto.2011.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GA, Beiser A, Rees TS, D’Agostino RB, & Wolf PA (2002). Central auditory dysfunction may precede the onset of clinical dementia in people with probable Alzheimer’s disease. J Am Geriatr Soc, 50(3), 482–488. doi: 10.1046/j.1532-5415.2002.50114.x [DOI] [PubMed] [Google Scholar]
- Gates GA, Karzon RK, Garcia P, Peterein J, Storandt M, Morris JC, & Miller JP (1995). Auditory dysfunction in aging and senile dementia of the Alzheimer’s type. Arch Neurol, 52(6), 626–634. doi: 10.1001/archneur.1995.00540300108020 [DOI] [PubMed] [Google Scholar]
- Gates GA, & Mills JH (2005). Presbycusis. Lancet, 366(9491), 1111–1120. doi: 10.1016/S0140-6736(05)67423-5 [DOI] [PubMed] [Google Scholar]
- Girard SD, Jacquet M, Baranger K, Migliorati M, Escoffier G, Bernard A, … Marchetti E (2014). Onset of hippocampus-dependent memory impairments in 5XFAD transgenic mouse model of Alzheimer’s disease. Hippocampus, 24(7), 762–772. doi: 10.1002/hipo.22267 [DOI] [PubMed] [Google Scholar]
- Glasberg BR, Moore BC, & Bacon SP (1987). Gap detection and masking in hearing-impaired and normal-hearing subjects. J Acoust Soc Am, 81(5), 1546–1556. doi: 10.1121/1.394507 [DOI] [PubMed] [Google Scholar]
- Grinberg LT, Rueb U, & Heinsen H (2011). Brainstem: neglected locus in neurodegenerative diseases. Front Neurol, 2, 42. doi: 10.3389/fneur.2011.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman EA, Bouter Y, Richard BC, Lannfelt L, Ingelsson M, Paetau A, … Bayer TA (2014). Abundance of Abeta(5)-x like immunoreactivity in transgenic 5XFAD, APP/PS1KI and 3xTG mice, sporadic and familial Alzheimer’s disease. Mol Neurodegener, 9, 13. doi: 10.1186/1750-1326-9-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggstrom J, Rosenhall U, Hederstierna C, Ostberg P, & Idrizbegovic E (2018). A Longitudinal Study of Peripheral and Central Auditory Function in Alzheimer’s Disease and in Mild Cognitive Impairment. Dement Geriatr Cogn Dis Extra, 8(3), 393–401. doi: 10.1159/000493340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrizbegovic E, Hederstierna C, Dahlquist M, Kampfe Nordstrom C, Jelic V, & Rosenhall U (2011). Central auditory function in early Alzheimer’s disease and in mild cognitive impairment. Age Ageing, 40(2), 249–254. doi: 10.1093/ageing/afq168 [DOI] [PubMed] [Google Scholar]
- Iliadou VV, Bamiou DE, Sidiras C, Moschopoulos NP, Tsolaki M, Nimatoudis I, & Chermak GD (2017). The Use of the Gaps-In-Noise Test as an Index of the Enhanced Left Temporal Cortical Thinning Associated with the Transition between Mild Cognitive Impairment and Alzheimer’s Disease. J Am Acad Audiol, 28(5), 463–471. doi: 10.3766/jaaa.16075 [DOI] [PubMed] [Google Scholar]
- Ison JR, O’Connor K, Bowen GP, & Bocirnea A (1991). Temporal resolution of gaps in noise by the rat is lost with functional decortication. Behav Neurosci, 105(1), 33–40. doi: 10.1037//0735-7044.105.1.33 [DOI] [PubMed] [Google Scholar]
- Jack CR Jr., & Holtzman DM (2013). Biomarker modeling of Alzheimer’s disease. Neuron, 80(6), 1347–1358. doi: 10.1016/j.neuron.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawhar S, Trawicka A, Jenneckens C, Bayer TA, & Wirths O (2012). Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Abeta aggregation in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol Aging, 33(1), 196 e129–140. doi: 10.1016/j.neurobiolaging.2010.05.027 [DOI] [PubMed] [Google Scholar]
- Kaczorowski CC, Sametsky E, Shah S, Vassar R, & Disterhoft JF (2011). Mechanisms underlying basal and learning-related intrinsic excitability in a mouse model of Alzheimer’s disease. Neurobiol Aging, 32(8), 1452–1465. doi: 10.1016/j.neurobiolaging.2009.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaylegian K, Stebritz AJ, Weible AP, & Wehr M (2019). 5XFAD Mice Show Early Onset Gap Detection Deficits. Front Aging Neurosci, 11, 66. doi: 10.3389/fnagi.2019.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JB, Rooney BJ, & Phillips DP (1996). Effects of bilateral auditory cortical lesions on gap-detection thresholds in the ferret (Mustela putorius). Behav Neurosci, 110(3), 542–550. doi: 10.1037//0735-7044.110.3.542 [DOI] [PubMed] [Google Scholar]
- Kimura R, & Ohno M (2009). Impairments in remote memory stabilization precede hippocampal synaptic and cognitive failures in 5XFAD Alzheimer mouse model. Neurobiol Dis, 33(2), 229–235. doi: 10.1016/j.nbd.2008.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen CM (2009). Nonparametric vs parametric tests of location in biomedical research. Am J Ophthalmol, 147(4), 571–572. doi: 10.1016/j.ajo.2008.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M (1999). The neurobiology of startle. Prog Neurobiol, 59(2), 107–128. doi: 10.1016/s0301-0082(98)00098-7 [DOI] [PubMed] [Google Scholar]
- Land R, Burghard A, & Kral A (2016). The contribution of inferior colliculus activity to the auditory brainstem response (ABR) in mice. Hear Res, 341, 109–118. doi: 10.1016/j.heares.2016.08.008 [DOI] [PubMed] [Google Scholar]
- Lee JH, Ryan J, Andreescu C, Aizenstein H, & Lim HK (2015). Brainstem morphological changes in Alzheimer’s disease. Neuroreport, 26(7), 411–415. doi: 10.1097/WNR.0000000000000362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhard W, & Lenhard A (2016). Calculation of Effect Sizes. Psychometrica. doi: 10.13140/RG.2.2.17823.92329 [DOI] [Google Scholar]
- Liu Y, Fang S, Liu LM, Zhu Y, Li CR, Chen K, & Zhao HB (2020). Hearing loss is an early biomarker in APP/PS1 Alzheimer’s disease mice. Neurosci Lett, 717, 134705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald IR, DeBay DR, Reid GA, O’Leary TP, Jollymore CT, Mawko G, … Darvesh S (2014). Early detection of cerebral glucose uptake changes in the 5XFAD mouse. Curr Alzheimer Res, 11(5), 450–460. doi: 10.2174/1567205011666140505111354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazi AR, Arzuman AS, Gurel B, Sahin B, Tuzuner MB, Ozansoy M, & Baykal AT (2018). Neonatal Neurodegeneration in Alzheimer’s Disease Transgenic Mouse Model. J Alzheimers Dis Rep, 2(1), 79–91. doi: 10.3233/ADR-170049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM (2018). Sex and Gender Differences in Alzheimer’s Disease Dementia. Psychiatr Times, 35(11), 14–17. [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Vemuri P, & Rocca WA (2014). Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin Epidemiol, 6, 37–48. doi: 10.2147/CLEP.S37929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmrich V, Grimm C, Draguhn A, Barghorn S, Lehmann A, Schoemaker H, … Bruehl C (2008). Amyloid beta oligomers (A beta(1–42) globulomer) suppress spontaneous synaptic activity by inhibition of P/Q-type calcium currents. J Neurosci, 28(4), 788–797. doi: 10.1523/JNEUROSCI.4771-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary TP, Shin S, Fertan E, Dingle RN, Almuklass A, Gunn RK, … Brown RE (2017). Reduced acoustic startle response and peripheral hearing loss in the 5xFAD mouse model of Alzheimer’s disease. Genes Brain Behav, 16(5), 554–563. doi: 10.1111/gbb.12370 [DOI] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, … Vassar R (2006). Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci, 26(40), 10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SJ, Lee HJ, Kang KJ, Han SJ, Lee YJ, Lee KC, … Choi JY (2018). Early Detection of Abeta Deposition in the 5xFAD Mouse by Amyloid PET. Contrast Media Mol Imaging, 2018, 5272014. doi: 10.1155/2018/5272014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, & Gagnon PM (2004). Apical-to-basal gradients in age-related cochlear degeneration and their relationship to “primary” loss of cochlear neurons. J Comp Neurol, 479(1), 103–116. doi: 10.1002/cne.20326 [DOI] [PubMed] [Google Scholar]
- Ohm TG, & Braak H (1989). Auditory brainstem nuclei in Alzheimer’s disease. Neurosci Lett, 96(1), 60–63. doi: 10.1016/0304-3940(89)90243-7 [DOI] [PubMed] [Google Scholar]
- Ohno M (2009). Failures to reconsolidate memory in a mouse model of Alzheimer’s disease. Neurobiol Learn Mem, 92(3), 455–459. doi: 10.1016/j.nlm.2009.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Chang L, Tseng W, Oakley H, Citron M, Klein WL, … Disterhoft JF (2006). Temporal memory deficits in Alzheimer’s mouse models: rescue by genetic deletion of BACE1. Eur J Neurosci, 23(1), 251–260. doi: 10.1111/j.1460-9568.2005.04551.x [DOI] [PubMed] [Google Scholar]
- Okanoya K, & Dooling RJ (1990). Detection of gaps in noise by budgerigars (Melopsittacus undulatus) and zebra finches (Poephila guttata). Hear Res, 50(1–2), 185–192. doi: 10.1016/0378-5955(90)90044-p [DOI] [PubMed] [Google Scholar]
- Parham K, & Willott JF (1990). Effects of inferior colliculus lesions on the acoustic startle response. Behav Neurosci, 104(6), 831–840. doi: 10.1037//0735-7044.104.6.831 [DOI] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen GW, & Damasio A (2001). The selective vulnerability of brainstem nuclei to Alzheimer’s disease. Ann Neurol, 49(1), 53–66. doi: [DOI] [PubMed] [Google Scholar]
- Plomp R (1964). The rate of decay of auditory senstaion. J Acoust Soc Am, 36, 277–282. doi: 10.1121/1.1918946 [DOI] [Google Scholar]
- Prange-Kiel J, Dudzinski DA, Prols F, Glatzel M, Matschke J, & Rune GM (2016). Aromatase Expression in the Hippocampus of AD Patients and 5xFAD Mice. Neural Plast, 2016, 9802086. doi: 10.1155/2016/9802086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish DA (2008). MClust spike sorting toolbox. Retrieved from http://redishlab.neuroscience.umn.edu/MClust/MClust.html
- Simic G, Stanic G, Mladinov M, Jovanov-Milosevic N, Kostovic I, & Hof PR (2009). Does Alzheimer’s disease begin in the brainstem? Neuropathol Appl Neurobiol, 35(6), 532–554. doi: 10.1111/j.1365-2990.2009.01038.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha UK, Hollen KM, Rodriguez R, & Miller CA (1993). Auditory system degeneration in Alzheimer’s disease. Neurology, 43(4), 779–785. doi: 10.1212/wnl.43.4.779 [DOI] [PubMed] [Google Scholar]
- Snell KB, & Frisina DR (2000). Relationships among age-related differences in gap detection and word recognition. J Acoust Soc Am, 107(3), 1615–1626. doi: 10.1121/1.428446 [DOI] [PubMed] [Google Scholar]
- Swords GM, Nguyen LT, Mudar RA, & Llano DA (2018). Auditory system dysfunction in Alzheimer disease and its prodromal states: A review. Ageing Res Rev, 44, 49–59. doi: 10.1016/j.arr.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana H, Takeda M, & Sugita M (1989). Brainstem auditory evoked potentials in patients with multi-infarct dementia and dementia of the Alzheimer type. Int J Neurosci, 48(3–4), 325–331. doi: 10.3109/00207458909002179 [DOI] [PubMed] [Google Scholar]
- Threlkeld SW, Penley SC, Rosen GD, & Fitch RH (2008). Detection of silent gaps in white noise following cortical deactivation in rats. Neuroreport, 19(8), 893–898. doi: 10.1097/WNR.0b013e3283013d7e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuwaig M, Savard M, Jutras B, Poirier J, Collins DL, Rosa-Neto P, … Group P-AR (2017). Deficit in Central Auditory Processing as a Biomarker of Pre-Clinical Alzheimer’s Disease. J Alzheimers Dis, 60(4), 1589–1600. doi: 10.3233/JAD-170545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese K, Molnar P, Das M, Bhargava N, Lambert S, Kindy MS, & Hickman JJ (2010). A new target for amyloid beta toxicity validated by standard and high-throughput electrophysiology. PLoS One, 5(1), e8643. doi: 10.1371/journal.pone.0008643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigts J (2013). Simpleclust - Manual spike sorting in matlab. Retrieved from http://jvoigts.scripts.mit.edu/blog/simpleclust-manual-spike-sorting-in-matlab/
- Wagner JM, Sichler ME, Schleicher EM, Franke TN, Irwin C, Low MJ, … Bouter Y (2019). Analysis of Motor Function in the Tg4–42 Mouse Model of Alzheimer’s Disease. Front Behav Neurosci, 13, 107. doi: 10.3389/fnbeh.2019.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weible AP, Liu C, Niell CM, & Wehr M (2014). Auditory cortex is required for fear potentiation of gap detection. J Neurosci, 34(46), 15437–15445. doi: 10.1523/JNEUROSCI.3408-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weible AP, Moore AK, Liu C, DeBlander L, Wu H, Kentros C, & Wehr M (2014). Perceptual gap detection is mediated by gap termination responses in auditory cortex. Curr Biol, 24(13), 1447–1455. doi: 10.1016/j.cub.2014.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weible AP, Yavorska I, & Wehr M (2020). A Cortico-Collicular Amplification Mechanism for Gap Detection. Cereb Cortex, 30(6), 3590–3607. doi: 10.1093/cercor/bhz328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirths O, Walter S, Kraus I, Klafki HW, Stazi M, Oberstein TJ, … Weggen S (2017). N-truncated Abeta4-x peptides in sporadic Alzheimer’s disease cases and transgenic Alzheimer mouse models. Alzheimers Res Ther, 9(1), 80. doi: 10.1186/s13195-017-0309-z [DOI] [PMC free article] [PubMed] [Google Scholar]


