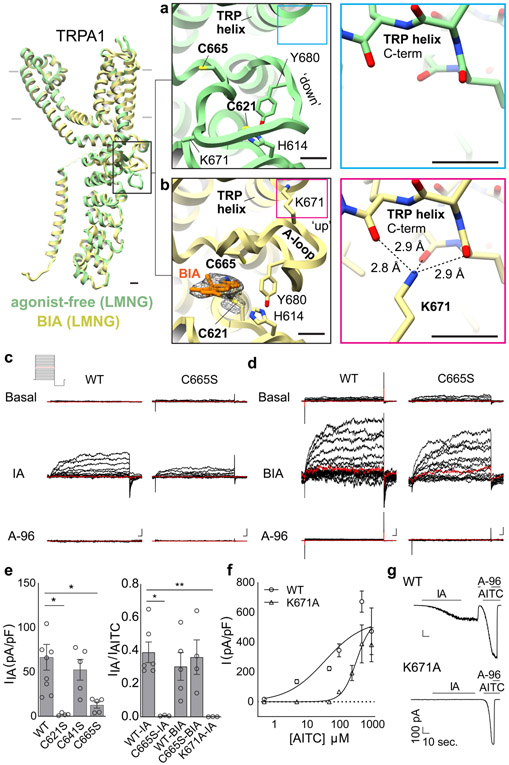
Fig. 3. Activation by electrophiles occurs through two-step mechanism.
a, A dynamic activation loop (A-loop) adopts a 'down' conformation in the agonist-free channel, partially occluding a reactive pocket containing C621. b, Following attachment of BODIPY-iodoacetamide (BIA) to C621, the A-loop transitions to an 'up' conformation, bringing C665 into the reactive pocket and repositioning K671 to coordinate backbone carbonyl oxygens at the TRP domain C-terminus. Scale bars: 5Å. c, IA (100 μM)-evoked currents for WT and C665S mutant TRPA1 channels (n = 9 and 5 independent experiments, respectively), followed by inhibition with A-96 (10 μM). d, Same for BIA (100 μM)-evoked currents (n = 5 for WT and C665S mutant). 500 msec. voltage steps from −80 to 80 mV, 0 mV colored red. Scale bars: x = 25 msec., y = 100 pA.
e, (left) Quantification of IA-evoked currents for WT or mutant TRPA1 channels at 80 mV (n = 9 and 5 independent experiments/construct, respectively). (right) Normalized IA-evoked currents (TRPA1 WT, n = 6; C665S, n = 3; K671A, n = 3) and BIA-evoked currents (TRPA1 WT n = 5; C665S, n =4). *, p = 0.05; ** p = 0.007, Kruskal-Wallis test with post-hoc Dunn’s correction for multiple comparisons. Data displayed as ± S.E.M. f, AITC dose-response curves for WT (EC50 = 37; 95% CI: 30 – 46 nM) and K671A (EC50 = 344; 95% CI: 313 – 381 nM) TRPA1 channels. EC50 values determined by nonlinear Poisson regression and statistically significant difference confirmed with extra sum-of-squares F test (p < 0.0001). n = 3 cells/dose/construct except K671A 50 mM (n = 6) and 100 mM (n = 4). Data are displayed as mean ± S.E.M. g, IA (100 μM) and AITC (1 mM)-evoked whole cell currents for WT and K671A channels (Vhold = −80 mV) blocked by A-96 (10 μM). n = 3 independent experiments/construct.