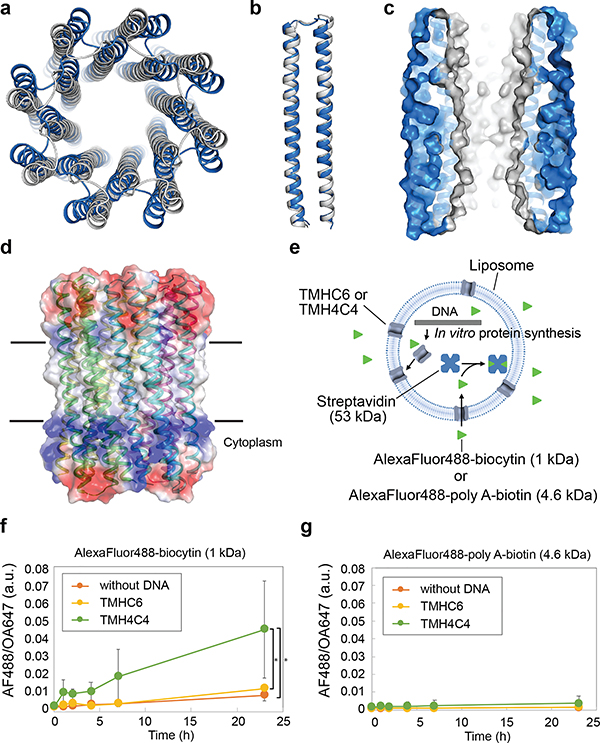
Figure 3. The X-ray crystal structure of water-soluble WSHC8 and the characterization of the 16-helix TMH4C4 transmembrane channel.
(a & b) Superposition of the full octameric assemblies and the monomeric subunits of the crystal structure (blue) and the design model (gray) of WSHC8. The C-ɑ RMSD is 2.51 Å and 0.97 Å, respectively. The larger deviation for the octamer is caused by the slight tilting of the hairpin monomers along the superhelical axis of the complex. (c) The cross section of the WSHC8 channel. (d) TMH4C4 model with 16 transmembrane helices. The electrostatic surface of the neutral transmembrane regions is in gray. (e) Liposome permeability assay. Membrane channels are generated by in vitro translation inside streptavidin containing liposomes, biotin-labeled fluorescent dyes are added to the surrounding buffer, and the amount of dye trapped inside the liposomes is measured by flow cytometry. (f & g) TMH4C4 functions as a size-dependent transmembrane sieve. Incorporation of TMH4C4 into liposomes allowed the accumulation of the 1 kDa but not 4.6 kDa fluorescent dye, while TMHC6 disallowed both. Time courses of the median values of the histogram of Alexa Fluor 488/Ovalbumin conjugated to Alexa Fluor 647 (AF488/OA647) fluorescence (Extended Data Fig. 6e) which represents the concentration of the Alexa Fluor 488 inside the liposome are shown. For (f & g), the error bars indicate s.e.m. (n = 7; the means of the obtained median values are shown). Student’s paired t-Test with a two-sided distribution was used to calculate the p values; *p=0.0128 (right) and 0.0220 (left). In control experiments performed with ɑ-hemolysin (AH) from Staphylococcus aureus, a well-studied channel forming protein33 with a pore constriction of ~15 Å; only the smaller dye accumulated (Extended Data Fig. 6f–g), suggesting that, as intended, the assay measures solute passage through the transmembrane pores.