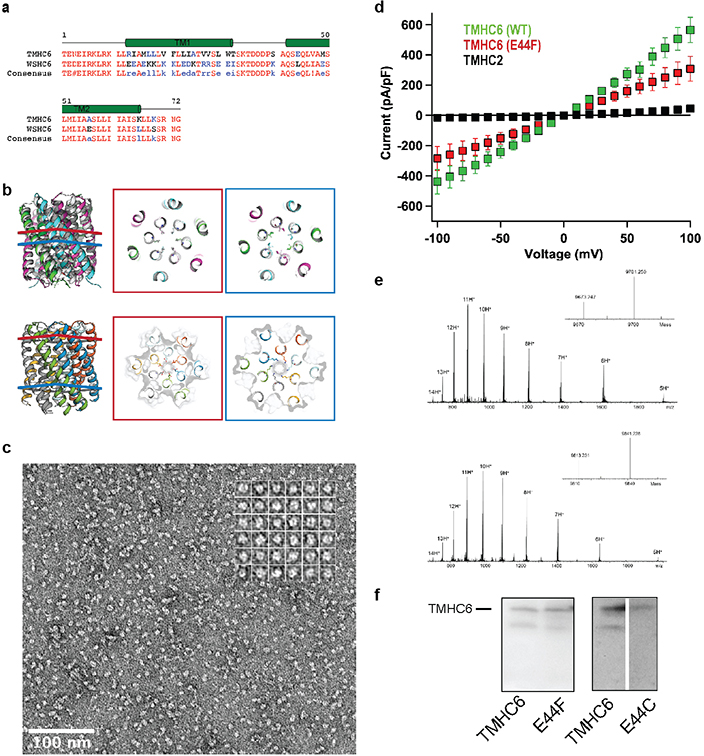
Extended Data Fig. 3 |. Comparisons between WSHC6 and TMHC6 and additional characterizations of TMHC6 and mutants.
(a) Sequence alignment of TMHC6 with WSHC6. (b) Pore-lining residues in WSHC6 and TMHC6. Top row: overlay of the crystal structure (colors) and the design model (gray) of WSHC6. The pore is lined with alternating leucine (red layer) and isoleucine (blue layer) residues. Bottom row: the TMHC6 pore is lined with E44 ring (red layer) and K65 ring (blue layer) at the extracellular and intracellular sides, respectively. (c) Negative stain EM for TMHC6 in amphipols. Protein particles on the EM grid showed round shape and size consistent with the design model (scale bar at the bottom left, 100 nm). Inset: close-up view of representative particles; each side of the particle frames represent 12.8 nm. (d) Disrupting mutation in the TMHC6 pore entrance reduces the current. The E44F single mutant reduced the K+ current to half of that for TMHC6. TMHC2, a previously designed transmembrane protein without a pore, does not conduct ions across the membrane. 3 cells were measured for each protein and the mean (data points) and standard error of measurement (s.e.m., error bars) are plotted. (e) The covalent modification of TMHC6 E44C mutant by MTSES. Mass-spectrometry results show that there is a 140 Da increase in molecular weight for the mutant after MTSES treatment, in agreement with the predicted value. (f) Expression of TMHC6 and mutants in insect cells for the whole-cell patch-clamp experiments. The same amount of cells were loaded into the gel and the expression levels for two variants were examined by western blot. The E44F mutant had similar, if not higher, expression level as TMHC6. The E44C mutant expressed at a slightly lower level compared to TMHC6. These experiments were repeated three times with similar results.